Page 93 - Read Online
P. 93
Ma et al. J Cancer Metastasis Treat 2022;8:25 https://dx.doi.org/10.20517/2394-4722.2022.17 Page 11 of 20
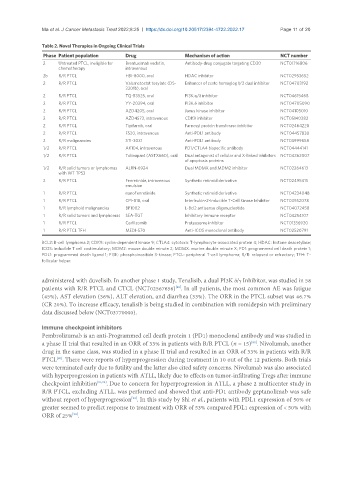
Table 2. Novel Therapies in Ongoing Clinical Trials
Phase Patient population Drug Mechanism of action NCT number
2 Untreated PTCL, ineligible for Brentuximab vedotin, Antibody-drug conjugate targeting CD30 NCT01716806
chemotherapy intravenous
2b R/R PTCL HBI-8000, oral HDAC inhibitor NCT02953652
2 R/R PTCL Valemetostat tosylate (DS- Enhancer of zeste homoglog 1/2 dual inhibitor NCT04703192
3201b), oral
2 R/R PTCL TQ-B3525, oral PI3K α/δ inhibitor NCT04615468
2 R/R PTCL YY-20394, oral PI3K δ inhibitor NCT04705090
2 R/R PTCL AZD4205, oral Janus kinase inhibitor NCT04105010
2 R/R PTCL AZD4573, intravenous CDK9 inhibitor NCT05140382
2 R/R PTCL Tipifarnib, oral Farnesyl protein transferase inhibitor NCT02464228
2 R/R PTCL F520, intravenous Anti-PDL1 antibody NCT04457830
2 R/R malignancies STI-3031 Anti-PDL1 antibody NCT03999658
1/2 R/R PTCL AK104, intravenous PD1/CTLA4 bispecific antibody NCT04444141
1/2 R/R PTCL Tolinapant (ASTX660), oral Dual antagonist of cellular and X-linked inhibitors NCT04362007
of apoptosis proteins
1/2 R/R solid tumors or lymphomas ALRN-6924 Dual MDMX and MDM2 inhibitor NCT02264613
with WT TP53
2 R/R PTCL Fenretinide, intravenous Synthetic retinoid derivative NCT02495415
emulsion
1 R/R PTCL nanoFenretinide Synthetic retinoid derivative NCT04234048
1 R/R PTCL CPI-818, oral Interleukin-2-Inducible T-Cell Kinase Inhibitor NCT03952078
1 R/R lymphoid malignancies BP1002 L-Bcl2 antisense oligonucleotide NCT04072458
1 R/R solid tumors and lymphomas SEA-TGT Inhibitory immune receptor NCT04254107
1 R/R PTCL Carfilzomib Proteosome inhibitor NCT01336920
1 R/R PTCL TFH MEDI-570 Anti-ICOS monoclonal antibody NCT02520791
BCL2: B-cell lymphoma 2; CDK9: cyclin-dependent kinase 9; CTLA4: cytotoxic T-lymphocyte-associated protein 4; HDAC: histone deacetylase;
ICOS: inducible T cell costimulatory; MDM2: mouse double minute 2; MDMX: murine double minute X; PD1: programmed cell death protein 1;
PDL1: programmed death ligand 1; PI3K: phosphoinositide 3-kinase; PTCL: peripheral T-cell lymphoma; R/R: relapsed or refractory; TFH: T-
follicular helper.
administered with duvelisib. In another phase 1 study, Tenalisib, a dual PI3K δ/γ Inhibitor, was studied in 58
[88]
patients with R/R PTCL and CTCL (NCT02567656) . In all patients, the most common AE was fatigue
(45%), AST elevation (36%), ALT elevation, and diarrhea (33%). The ORR in the PTCL subset was 46.7%
(CR 20%). To increase efficacy, tenalisib is being studied in combination with romidepsin with preliminary
data discussed below (NCT03770000).
Immune checkpoint inhibitors
Pembrolizumab is an anti-Programmed cell death protein 1 (PD1) monoclonal antibody and was studied in
[89]
a phase II trial that resulted in an ORR of 33% in patients with R/R PTCL (n = 15) . Nivolumab, another
drug in the same class, was studied in a phase II trial and resulted in an ORR of 33% in patients with R/R
[90]
PTCL . There were reports of hyperprogression during treatment in 10 out of the 12 patients. Both trials
were terminated early due to futility and the latter also cited safety concerns. Nivolumab was also associated
with hyperprogression in patients with ATLL, likely due to effects on tumor-infiltrating Tregs after immune
checkpoint inhibition [91,92] . Due to concern for hyperprogression in ATLL, a phase 2 multicenter study in
R/R PTCL, excluding ATLL, was performed and showed that anti-PD1 antibody geptanolimab was safe
[93]
without report of hyperprogression . In this study by Shi et al., patients with PDL1 expression of 50% or
greater seemed to predict response to treatment with ORR of 53% compared PDL1 expression of < 50% with
ORR of 25% .
[93]