Page 91 - Read Online
P. 91
Ma et al. J Cancer Metastasis Treat 2022;8:25 https://dx.doi.org/10.20517/2394-4722.2022.17 Page 9 of 20
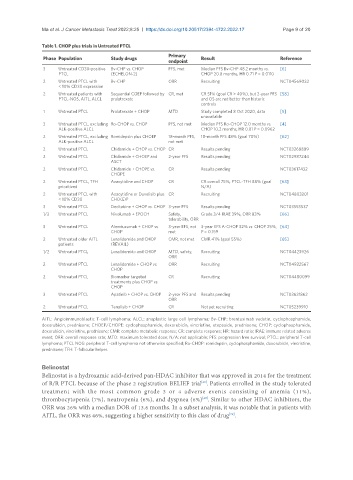
Table 1. CHOP plus trials in Untreated PTCL
Primary
Phase Population Study drugs Result Reference
endpoint
3 Untreated CD30-positive Bv-CHP vs. CHOP PFS, met Median PFS Bv-CHP 48.2 months vs. [6]
PTCL (ECHELON-2) CHOP 20.8 months, HR 0.71 P = 0.0110
2 Untreated PTCL with Bv-CHP ORR Recruiting NCT04569032
< 10% CD30 expression
2 Untreated patients with Sequential COEP followed by CR, met CR 51% (goal CR > 40%), but 2-year PFS [58]
PTCL-NOS, AITL, ALCL pralatrexate and OS are not better than historic
controls
1 Untreated PTCL Pralatrexate + CHOP MTD Study completed 8 Oct 2020, data [5]
unavailable
3 Untreated PTCL, excluding Ro-CHOP vs. CHOP PFS, not met Median PFS Ro-CHOP 12.0 months vs. [4]
ALK-positive ALCL CHOP 10.2 months, HR 0.81 P = 0.0962
2 Untreated PTCL, excluding Romidepsin plus CHOEP 18-month PFS, 18-month PFS 48% (goal 70%) [62]
ALK-positive ALCL not met
2 Untreated PTCL Chidamide + CHOP vs. CHOP CR Results pending NCT03268889
2 Untreated PTCL Chidamide + CHOEP and 2-year PFS Results pending NCT02987244
ASCT
2 Untreated PTCL Chidamide + CHOPE vs. CR Results pending NCT03617432
CHOPE
2 Untreated PTCL, TFH Azacytidine and CHOP CR CR overall 75%, PTCL-TFH 88% (goal [63]
prioritized N/A)
2 Untreated PTCL with Azacytidine or Duvelisib plus CR Recruiting NCT04803201
< 10% CD30 CHO(E)P
3 Untreated PTCL Decitabine + CHOP vs. CHOP 3-year PFS Results pending NCT03553537
1/2 Untreated PTCL Nivolumab + EPOCH Safety, Grade 3/4 IRAE 39%, ORR 83% [66]
tolerability, ORR
3 Untreated PTCL Alemtuzumab + CHOP vs. 3-year EFS, not 3-year EFS A-CHOP 32% vs. CHOP 25%, [64]
CHOP met P = 0.159
2 Untreated older AITL Lenalidomide and CHOP CMR, not met CMR 41% (goal 55%) [65]
patients (REVAIL)
1/2 Untreated PTCL Lenalidomide and CHOP MTD, safety, Recruiting NCT04423926
ORR
2 Untreated PTCL Lenalidomide + CHOP vs. ORR Recruiting NCT04922567
CHOP
2 Untreated PTCL Biomarker targeted CR Recruiting NCT04480099
treatments plus CHOP vs.
CHOP
3 Untreated PTCL Apatinib + CHOP vs. CHOP 2-year PFS and Results pending NCT03631862
ORR
2 Untreated PTCL Tenalisib + CHOP CR Not yet recruiting NCT05239910
AITL: Angioimmunoblastic T-cell lymphoma; ALCL: anaplastic large cell lymphoma; Bv-CHP: brentuximab vedotin, cyclophosphamide,
doxorubicin, prednisone; CHOEP/CHOPE: cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; CHOP: cyclophosphamide,
doxorubicin, vincristine, prednisone; CMR: complete metabolic response; CR: complete response; HR: hazard ratio; IRAE: immune related adverse
event; ORR: overall response rate; MTD: maximum tolerated dose; N/A: not applicable; PFS: progression free survival; PTCL: peripheral T-cell
lymphoma; PTCL NOS: peripheral T-cell lymphoma not otherwise specified; Ro-CHOP: romidepsin, cyclophosphamide, doxorubicin, vincristine,
prednisone; TFH: T-follicular helper.
Belinostat
Belinostat is a hydroxamic acid-derived pan-HDAC inhibitor that was approved in 2014 for the treatment
[69]
of R/R PTCL because of the phase 2 registration BELIEF trial . Patients enrolled in the study tolerated
treatment with the most common grade 3 or 4 adverse events consisting of anemia (11%),
[69]
thrombocytopenia (7%), neutropenia (6%), and dyspnea (6%) . Similar to other HDAC inhibitors, the
ORR was 26% with a median DOR of 13.6 months. In a subset analysis, it was notable that in patients with
[79]
AITL, the ORR was 46%, suggesting a higher sensitivity to this class of drug .