Page 97 - Read Online
P. 97
Ma et al. J Cancer Metastasis Treat 2022;8:25 https://dx.doi.org/10.20517/2394-4722.2022.17 Page 15 of 20
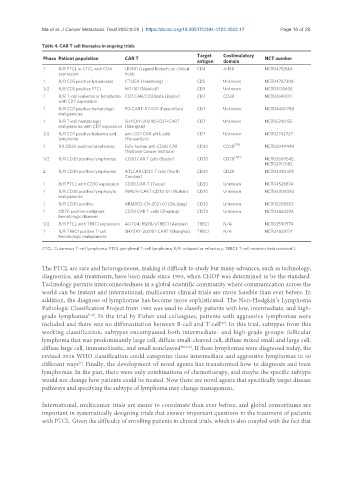
Table 4. CAR T cell therapies in ongoing trials
Target Costimulatory
Phase Patient population CAR T NCT number
antigen domain
1 R/R PTCL or CTCL with CD4 LB1901 (Legend Biotech, on clinical CD4 4-1BB NCT04712864
expression hold)
1 R/R CD5 positive lymphomas CT125A (Huazhong) CD5 Unknown NCT04767308
1/2 R/R CD5 positive PTCL MT-101 (Myeloid) CD5 Unknown NCT05138458
1 R/R T-cell leukemia or lymphoma CD7.CAR/CD28zeta (Baylor) CD7 CD28 NCT03690011
with CD7 expression
1 R/R CD7 positive hematologic PG-CART-07-001 (PersonGen) CD7 Unknown NCT04480788
malignancies
1 R/R T-cell hematologic SHYSXY-202105-CD7-CART CD7 Unknown NCT05290155
malignancies with CD7 expression (Shanghai)
1/2 R/R CD7 positive leukemia and anti-CD7 CAR-pNK cells CD7 Unknown NCT02742727
lymphoma (PersonGen)
1 R.R CD30 positive lymphomas Fully human anti-CD30 CAR CD30 CD28 [113] NCT03049449
(National Cancer Institute)
1/2 R/R CD30 positive lymphomas CD30.CAR T cells (Baylor) CD30 CD28 [114] NCT02690545,
NCT02917083
2 R/R CD30 positive lymphomas ATLCAR.CD30 T cells (North CD30 CD28 NCT04083495
Carolina)
1 R/R PTCL with CD30 expression CD30.CAR-T (Tessa) CD30 Unknown NCT04526834
1 R/R CD30 positive lymphocyte WHUH-CART-CD30-01 (Wuhan) CD30 Unknown NCT04008394
malignancies
1 R/R CD30 positive ARM002-CN-2021-01 (Zhejiang) CD30 Unknown NCT05208853
1 CD70 positive malignant CD70 CAR T-cells (Zhejiang) CD70 Unknown NCT04662294
hematologic diseases
1/2 R/R PTCL with TRBC1 expression AUTO4, RQR8/aTRBC1 (Autolus) TRBC1 N/A NCT03590574
1 R/R TRBC1 positive T-cell SHYSXY-202101-CART (Shanghai) TRBC1 N/A NCT04828174
hematologic malignancies
CTCL: Cutaneous T-cell lymphoma; PTCL: peripheral T-cell lymphoma; R/R: relapsed or refractory; TRBC1: T-cell receptor beta constant 1.
The PTCL are rare and heterogeneous, making it difficult to study but many advances, such as technology,
diagnostics, and treatments, have been made since 1993, when CHOP was determined to be the standard.
Technology permits interconnectedness in a global scientific community where communication across the
world can be instant and international, multicenter clinical trials are more feasible than ever before. In
addition, the diagnose of lymphomas has become more sophisticated. The Non-Hodgkin’s Lymphoma
Pathologic Classification Project from 1982 was used to classify patients with low, intermediate, and high-
[112]
grade lymphomas . In the trial by Fisher and colleagues, patients with aggressive lymphomas were
included and there was no differentiation between B-cell and T-cell . In this trial, subtypes from this
[48]
working classification, subtypes encompassed both intermediate- and high-grade groups: follicular
lymphoma that was predominantly large cell, diffuse small-cleaved cell, diffuse mixed small and large cell,
diffuse large cell, immunoblastic, and small noncleaved [89,112] . If these lymphomas were diagnosed today, the
revised 2016 WHO classification could categorize these intermediate and aggressive lymphomas in 50
different ways . Finally, the development of novel agents has transformed how to diagnosis and treat
[1]
lymphomas. In the past, there were only combinations of chemotherapy, and maybe the specific subtype
would not change how patients could be treated. Now there are novel agents that specifically target disease
pathways and specifying the subtype of lymphoma may change management.
International, multicenter trials are easier to coordinate than ever before, and global consortiums are
important in systematically designing trials that answer important questions in the treatment of patients
with PTCL. Given the difficulty of enrolling patients in clinical trials, which is also coupled with the fact that