Page 110 - Read Online
P. 110
Davidson et al. J Cancer Metastasis Treat 2021;7:45 https://dx.doi.org/10.20517/2394-4722.2021.77 Page 13 of 19
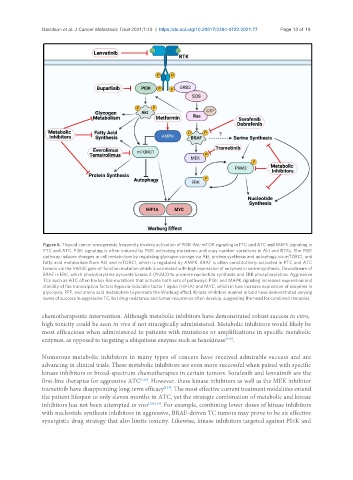
Figure 6. Thyroid cancer oncogenesis frequently invokes activation of PI3K-Akt-mTOR signaling in FTC and ATC and MAPK signaling in
PTC and ATC. PI3K signaling is often induced by PI3K activating mutations and copy number variations in Akt and RTKs. The PI3K
pathway induces changes in cell metabolism by regulating glycogen storage via Akt, protein synthesis and autophagy via mTORC1, and
fatty acid metabolism from Akt and mTORC1, which is regulated by AMPK. BRAF is often constitutively activated in PTC and ATC
tumors via the V600E gain-of-function mutation which is correlated with high expression of enzymes in serine synthesis. Downstream of
BRAF is ERK, which phosphorylates pyruvate kinase 2 (PKM2) to promote nucleotide synthesis and ERK phosphorylation. Aggressive
TCs such as ATC often harbor Ras mutations that activate both sets of pathways. PI3K and MAPK signaling increases expression and
stability of the transcription factors hypoxia-inducible factor 1 alpha (HIF1A) and MYC, which in turn increase expression of enzymes in
glycolysis, PPP, and amino acid metabolism to promote the Warburg effect. Kinase inhibitors marked in bold have demonstrated varying
levels of success in aggressive TC, but drug resistance and tumor recurrence often develop, suggesting the need for combined therapies.
chemotherapeutic intervention. Although metabolic inhibitors have demonstrated robust success in vitro,
high toxicity could be seen in vivo if not strategically administered. Metabolic inhibitors would likely be
most efficacious when administered to patients with mutations or amplifications in specific metabolic
enzymes, as opposed to targeting a ubiquitous enzyme such as hexokinase .
[156]
Numerous metabolic inhibitors in many types of cancers have received admirable success and are
advancing in clinical trials. These metabolic inhibitors are even more successful when paired with specific
kinase inhibitors or broad-spectrum chemotherapies in certain tumors. Sorafenib and lenvatinib are the
first-line therapies for aggressive ATC [3,10] . However, these kinase inhibitors as well as the MEK inhibitor
[157]
trametinib have disappointing long-term efficacy . The most effective current treatment modalities extend
the patient lifespan to only eleven months in ATC, yet the strategic combination of metabolic and kinase
inhibitors has not been attempted in vivo [158,159] . For example, combining lower doses of kinase inhibitors
with nucleotide synthesis inhibitors in aggressive, BRAF-driven TC tumors may prove to be an effective
synergistic drug strategy that also limits toxicity. Likewise, kinase inhibitors targeted against PI3K and