Page 103 - Read Online
P. 103
Tessari et al. J Cancer Metastasis Treat 2020;6:18 I http://dx.doi.org/10.20517/2394-4722.2020.32 Page 7 of 11
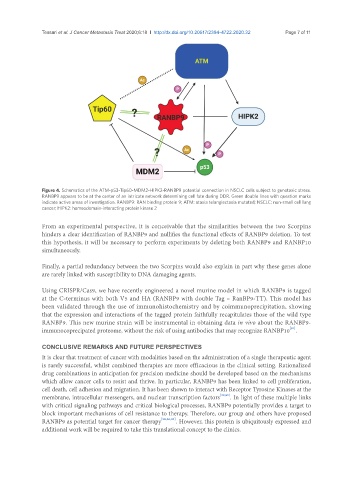
Figure 4. Schematics of the ATM-p53-Tip60-MDM2-HIPK2-RANBP9 potential connection in NSCLC cells subject to genotoxic stress.
RANBP9 appears to be at the center of an intricate network determining cell fate during DDR. Green double lines with question marks
indicate active areas of investigation. RANBP9: RAN binding protein 9; ATM: ataxia telangiectasia mutated; NSCLC: non-small cell lung
cancer; HIPK2: homeodomain-interacting protein kinase 2
From an experimental perspective, it is conceivable that the similarities between the two Scorpins
hinders a clear identification of RANBP9 and nullifies the functional effects of RANBP9 deletion. To test
this hypothesis, it will be necessary to perform experiments by deleting both RANBP9 and RANBP10
simultaneously.
Finally, a partial redundancy between the two Scorpins would also explain in part why these genes alone
are rarely linked with susceptibility to DNA damaging agents.
Using CRISPR/Cas9, we have recently engineered a novel murine model in which RANBP9 is tagged
at the C-terminus with both V5 and HA (RANBP9 with double Tag = RanBP9-TT). This model has
been validated through the use of immunohistochemistry and by coimmunoprecipitation, showing
that the expression and interactions of the tagged protein faithfully recapitulates those of the wild type
RANBP9. This new murine strain will be instrumental in obtaining data in vivo about the RANBP9-
[84]
immunocoprecipated proteome, without the risk of using antibodies that may recognize RANBP10 .
CONCLUSIVE REMARKS AND FUTURE PERSPECTIVES
It is clear that treatment of cancer with modalities based on the administration of a single therapeutic agent
is rarely successful, whilst combined therapies are more efficacious in the clinical setting. Rationalized
drug combinations in anticipation for precision medicine should be developed based on the mechanisms
which allow cancer cells to resist and thrive. In particular, RANBP9 has been linked to cell proliferation,
cell death, cell adhesion and migration. It has been shown to interact with Receptor Tyrosine Kinases at the
membrane, intracellular messengers, and nuclear transcription factors [30,85] . In light of these multiple links
with critical signaling pathways and critical biological processes, RANBP9 potentially provides a target to
block important mechanisms of cell resistance to therapy. Therefore, our group and others have proposed
RANBP9 as potential target for cancer therapy [50,56,85] . However, this protein is ubiquitously expressed and
additional work will be required to take this translational concept to the clinics.