Page 100 - Read Online
P. 100
Page 4 of 11 Tessari et al. J Cancer Metastasis Treat 2020;6:18 I http://dx.doi.org/10.20517/2394-4722.2020.32
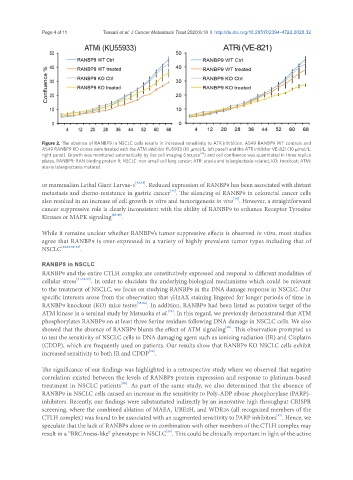
Figure 2. The absence of RANBP9 in NSCLC cells results in increased sensitivity to ATR inhibition. A549 RANBP9 WT controls and
A549 RANBP9 KO clones were treated with the ATM inhibitor KU5933 (10 μmol/L; left panel) and the ATR inhibitor VE-821 (10 μmol/L;
TM
right panel). Growth was monitored automatically by live cell imaging (Incuyte ) and cell confluence was quantitated in three replica
plates. RANBP9: RAN binding protein 9; NSCLC: non-small cell lung cancer; ATR: ataxia and telangiectasia-related; KO: knockout; ATM:
ataxia telangiectasia mutated
or mammalian Lethal Giant Larvae-1 [42,43] . Reduced expression of RANBP9 has been associated with distant
[44]
metastasis and chemo-resistance in gastric cancer . The silencing of RANBP9 in colorectal cancer cells
[45]
also resulted in an increase of cell growth in vitro and tumorigenesis in vivo . However, a straightforward
cancer suppressive role is clearly inconsistent with the ability of RANBP9 to enhance Receptor Tyrosine
Kinases or MAPK signaling [46-49] .
While it remains unclear whether RANBP9’s tumor suppressive effects is observed in vitro, most studies
agree that RANBP9 is over-expressed in a variety of highly prevalent tumor types including that of
NSCLC [44,45,50-52] .
RANBP9 in NSCLC
RANBP9 and the entire CTLH complex are constitutively expressed and respond to different modalities of
cellular stress [31,32,53] . In order to elucidate the underlying biological mechanisms which could be relevant
to the treatment of NSCLC, we focus on studying RANBP9 in the DNA damage response in NSCLC. Our
specific interests arose from the observation that gH2AX staining lingered for longer periods of time in
RANBP9 knockout (KO) mice testes [35,54] . In addition, RANBP9 had been listed as putative target of the
[55]
ATM kinase in a seminal study by Matsuoka et al. . In this regard, we previously demonstrated that ATM
phosphorylates RANBP9 on at least three Serine residues following DNA damage in NSCLC cells. We also
[56]
showed that the absence of RANBP9 blunts the effect of ATM signaling . This observation prompted us
to test the sensitivity of NSCLC cells to DNA damaging agent such as ionizing radiation (IR) and Cisplatin
(CDDP), which are frequently used on patients. Our results show that RANBP9 KO NSCLC cells exhibit
[56]
increased sensitivity to both IR and CDDP .
The significance of our findings was highlighted in a retrospective study where we observed that negative
correlation existed between the levels of RANBP9 protein expression and response to platinum-based
[50]
treatment in NSCLC patients . As part of the same study, we also determined that the absence of
RANBP9 in NSCLC cells caused an increase in the sensitivity to Poly-ADP ribose phosphorylase (PARP)-
inhibitors. Recently, our findings were substantiated indirectly by an innovative high throughput CRISPR
screening, where the combined ablation of MAEA, UBE2H, and WDR26 (all recognized members of the
CTLH complex) was found to be associated with an augmented sensitivity to PARP inhibitors . Hence, we
[57]
speculate that the lack of RANBP9 alone or in combination with other members of the CTLH complex may
result in a “BRCAness-like” phenotype in NSCLC . This could be clinically important in light of the active
[58]