Page 42 - Read Online
P. 42
Liu et al. J Cancer Metastasis Treat 2019;5:4 I http://dx.doi.org/10.20517/2394-4722.2018.55 Page 9 of 14
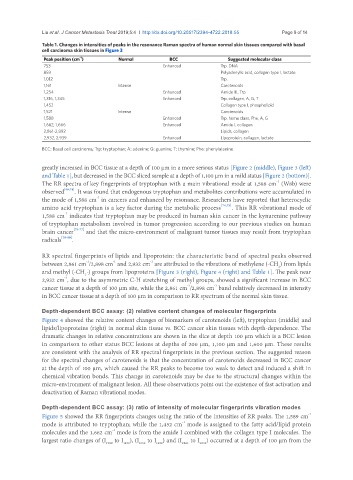
Table 1. Changes in intensities of peaks in the resonance Raman spectra of human normal skin tissues compared with basal
cell carcinoma skin tissues in Figure 3
-1
Peak position (cm ) Normal BCC Suggested molecular class
753 Enhanced Trp. DNA
859 Polyadenylic acid, collagen type I, lactate
1,012 Trp.
1,161 Intense Carotenoids
1,254 Enhanced Amide III, Trp.
1,316, 1,345 Enhanced Trp. collagen, A, G, T
1,452 Collagen type I, phospholipid
1,521 Intense Carotenoids
1,588 Enhanced Trp. heme class, Phe, A, G
1,662, 1,666 Enhanced Amide I, collagen
2,861-2,892 Lipids, collagen
2,932, 2,939 Enhanced Lipoprotein, collagen, lactate
BCC: Basal cell carcinoma; Trp: tryptophan; A: adenine; G: guanine; T: thymine; Phe: phenylalanine
greatly increased in BCC tissue at a depth of 100 µm in a more serious status [Figure 2 (middle), Figure 3 (left)
and Table 1], but decreased in the BCC sliced sample at a depth of 1,100 µm in a mild status [Figure 2 (bottom)].
The RR spectra of key fingerprints of tryptophan with a main vibrational mode at 1,588 cm (W8b) were
-1
observed [66,74] . It was found that endogenous tryptophan and metabolites contributions were accumulated in
-1
the mode of 1,588 cm in cancers and enhanced by resonance. Researchers have reported that heterocyclic
amino acid tryptophan is a key factor during the metabolic process [74,75] . This RR vibrational mode of
-1
1,588 cm indicates that tryptophan may be produced in human skin cancer in the kynurenine pathway
of tryptophan metabolism involved in tumor progression according to our previous studies on human
brain cancer [75-77] and that the micro-environment of malignant tumor tissues may result from tryptophan
radicals [78-80] .
RR spectral fingerprints of lipids and lipoprotein: the characteristic band of spectral peaks observed
-1
-1
-1
between 2,861 cm /2,898 cm and 2,932 cm are attributed to the vibrations of methylene (-CH ) from lipids
2
and methyl (-CH -) groups from lipoproteins [Figure 3 (right), Figure 4 (right) and Table 1]. The peak near
3
2,932 cm , due to the asymmetric C-H stretching of methyl groups, showed a significant increase in BCC
-1
-1
-1
cancer tissue at a depth of 100 µm site, while the 2,861 cm /2,898 cm band relatively decreased in intensity
in BCC cancer tissue at a depth of 100 µm in comparison to RR spectrum of the normal skin tissue.
Depth-dependent BCC assay: (2) relative content changes of molecular fingerprints
Figure 4 showed the relative content changes of biomarkers of carotenoids (left), tryptophan (middle) and
lipids/lipoproteins (right) in normal skin tissue vs. BCC cancer skin tissues with depth-dependence. The
dramatic changes in relative concentrations are shown in the slice at depth 100 µm which is a BCC lesion
in comparison to other status BCC lesions at depths of 200 µm, 1,100 µm and 1,600 µm. These results
are consistent with the analysis of RR spectral fingerprints in the previous section. The suggested reason
for the spectral changes of carotenoids is that the concentration of carotenoids decreased in BCC cancer
at the depth of 100 µm, which caused the RR peaks to become too weak to detect and induced a shift in
chemical vibration bonds. This change in carotenoids may be due to the structural changes within the
micro-environment of malignant lesion. All these observations point out the existence of fast activation and
deactivation of Raman vibrational modes.
Depth-dependent BCC assay: (3) ratio of intensity of molecular fingerprints vibration modes
-1
Figure 5 showed the RR fingerprints changes using the ratio of the intensities of RR peaks. The 1,589 cm
-1
mode is attributed to tryptophan; while the 1,452 cm mode is assigned to the fatty acid/lipid protein
-1
molecules and the 1,662 cm mode is from the amide I combined with the collagen type I molecules. The
largest ratio changes of (I to I ), (I to I ) and (I to I ) occurred at a depth of 100 µm from the
1452
1589
2861
1452
2932
1662