Page 61 - Read Online
P. 61
Chi et al. J Cancer Metastasis Treat 2020;6:43 I http://dx.doi.org/10.20517/2394-4722.2020.90 Page 3 of 17
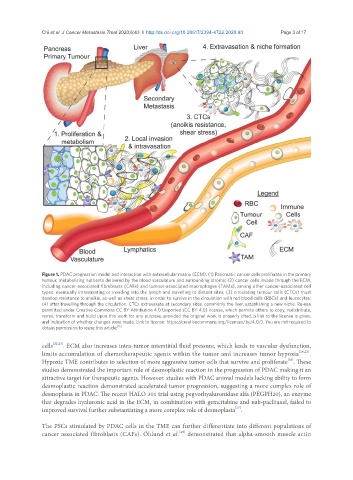
Figure 1. PDAC progression model and interaction with extracellular matrix (ECM). (1) Pancreatic cancer cells proliferate in the primary
tumour, metabolising nutrients delivered by the blood vasculature and surrounding stroma; (2) cancer cells invade through the ECM,
including cancer-associated fibroblasts (CAFs) and tumour-associated macrophages (TAMs), among other cancer-associated cell
types, eventually intravasating or invading into the lymph and travelling to distant sites; (3) circulating tumour cells (CTCs) must
develop resistance to anoikis, as well as shear stress, in order to survive in the circulation with red blood cells (RBCs) and leucocytes;
(4) after travelling through the circulation, CTCs extravasate at secondary sites, commonly the liver, establishing a new niche. Re-use
permitted under Creative Commons CC BY Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute,
remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the license is given,
and indication of whether changes were made. Link to license: https://creativecommons.org/licenses/by/4.0/). You are not required to
obtain permission to reuse this article [18]
cells [22,23] . ECM also increases intra-tumor interstitial fluid pressure, which leads to vascular dysfunction,
limits accumulation of chemotherapeutic agents within the tumor and increases tumor hypoxia [24,25] .
[26]
Hypoxic TME contributes to selection of more aggressive tumor cells that survive and proliferate . These
studies demonstrated the important role of desmoplastic reaction in the progression of PDAC making it an
attractive target for therapeutic agents. However, studies with PDAC animal models lacking ability to form
desmoplastic reaction demonstrated accelerated tumor progression, suggesting a more complex role of
desmoplasia in PDAC. The recent HALO 301 trial using pegvorhyaluronidase alfa (PEGPH20), an enzyme
that degrades hyaluronic acid in the ECM, in combination with gemcitabine and nab-paclitaxel, failed to
[27]
improved survival further substantiating a more complex role of desmoplasia .
The PSCs stimulated by PDAC cells in the TME can further differentiate into different populations of
[28]
cancer associated fibroblasts (CAFs). Öhlund et al. demonstrated that alpha-smooth muscle actin