Page 65 - Read Online
P. 65
Chi et al. J Cancer Metastasis Treat 2020;6:43 I http://dx.doi.org/10.20517/2394-4722.2020.90 Page 7 of 17
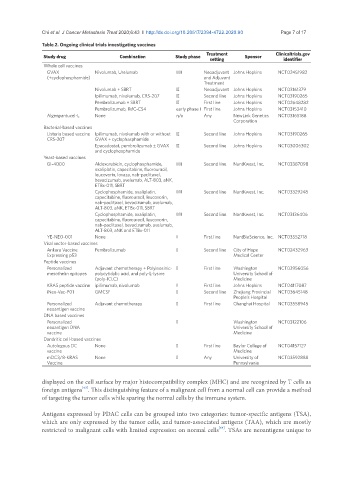
Table 2. Ongoing clinical trials investigating vaccines
Treatment Clinicaltrials.gov
Study drug Combination Study phase Sponsor
setting identifier
Whole cell vaccines
GVAX Nivolumab, Urelumab I/II Neoadjuvant Johns Hopkins NCT02451982
(+cyclophosphamide) and Adjuvant
Treatment
Nivolumab + SBRT II Neoadjuvant Johns Hopkins NCT03161379
Ipilimumab, nivolumab, CRS-207 II Second line Johns Hopkins NCT03190265
Pembrolizumab + SBRT II First line Johns Hopkins NCT02648282
Pembrolizumab, IMC-CS4 early phase I First line Johns Hopkins NCT03153410
Algenpantucel-L None n/a Any NewLink Genetics NCT03165188
Corporation
Bacterial-based vaccines
Listeria based vaccine Ipilimumab, nivolumab with or without II Second line Johns Hopkins NCT03190265
CRS-207 GVAX + cyclophasphamide
Epacadostat, pembrolizumab ± GVAX II Second line Johns Hopkins NCT03006302
and cyclophosphamide
Yeast-based vaccines
GI-4000 Aldoxorubicin, cyclophosphamide, I/II Second line NantKwest, Inc. NCT03387098
oxaliplatin, capecitabine, fluorouracil,
leucovorin, lovaza, nab-paclitaxel,
bevacizumab, avelumab, ALT-803, aNK,
ETBx-011, SBRT
Cyclophosphamide, oxaliplatin, I/II Second line NantKwest, Inc. NCT03329248
capecitabine, fluorouracil, leucovorin,
nab-paclitaxel, bevacizumab, avelumab,
ALT-803, aNK, ETBx-011, SBRT
Cyclophosphamide, oxaliplatin, I/II Second line NantKwest, Inc. NCT03136406
capecitabine, fluorouracil, leucovorin,
nab-paclitaxel, bevacizumab, avelumab,
ALT-803, aNK and ETBx-011
YE-NEO-001 None I First line NantBioScience, Inc. NCT03552718
Viral vector-based vaccines
Ankara Vaccine Pembrolizumab I Second line City of Hope NCT02432963
Expressing p53 Medical Center
Peptide vaccines
Personalized Adjuvant chemotherapy + Polyinosinic- I First line Washington NCT03956056
mesothelin epitopes polycytidylic acid, and poly-L-lysine University School of
(poly-ICLC) Medicine
KRAS peptide vaccine ipilimumab, nivolumab I First line Johns Hopkins NCT04117087
iNeo-Vac-P01 GMCSF I Second line Zhejiang Provincial NCT03645148
People’s Hospital
Personalized Adjuvant chemotherapy I First line Changhai Hospital NCT03558945
neoantigen vaccine
DNA based vaccines
Personalized I Washington NCT03122106
neoantigen DNA University School of
vaccine Medicine
Dandritic cell-based vaccines
Autologous DC None I First line Baylor College of NCT04157127
vaccine Medicine
mDC3/8-KRAS None I Any University of NCT03592888
Vaccine Pennsylvania
displayed on the cell surface by major histocompatibility complex (MHC) and are recognized by T cells as
foreign antigens . This distinguishing feature of a malignant cell from a normal cell can provide a method
[63]
of targeting the tumor cells while sparing the normal cells by the immune system.
Antigens expressed by PDAC cells can be grouped into two categories: tumor-specific antigens (TSA),
which are only expressed by the tumor cells, and tumor-associated antigens (TAA), which are mostly
[64]
restricted to malignant cells with limited expression on normal cells . TSAs are neoantigens unique to