Page 67 - Read Online
P. 67
Pippione et al. Steroidogenic enzymes in prostate cancer
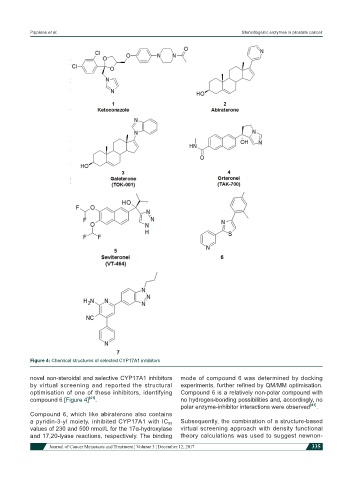
Figure 4: Chemical structures of selected CYP17A1 inhibitors
novel non-steroidal and selective CYP17A1 inhibitors mode of compound 6 was determined by docking
by virtual screening and reported the structural experiments, further refined by QM/MM optimisation.
optimisation of one of these inhibitors, identifying Compound 6 is a relatively non-polar compound with
compound 6 [Figure 4] [41] . no hydrogen-bonding possibilities and, accordingly, no
polar enzyme-inhibitor interactions were observed [41] .
Compound 6, which like abiraterone also contains
Subsequently, the combination of a structure-based
a pyridin-3-yl moiety, inhibited CYP17A1 with IC 50
values of 230 and 500 nmol/L for the 17α-hydroxylase virtual screening approach with density functional
and 17,20-lyase reactions, respectively. The binding theory calculations was used to suggest newnon-
Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017 335