Page 66 - Read Online
P. 66
Pippione et al. Steroidogenic enzymes in prostate cancer
A
B
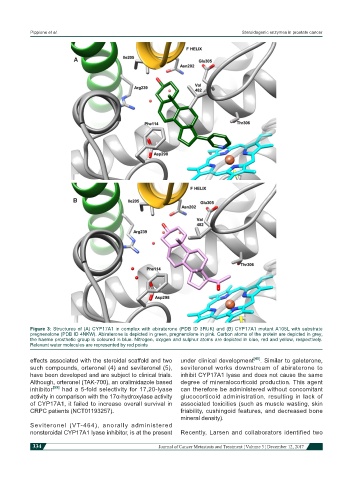
Figure 3: Structures of (A) CYP17A1 in complex with abiraterone (PDB ID 3RUK) and (B) CYP17A1 mutant A105L with substrate
pregnenolone (PDB ID 4NKW). Abiraterone is depicted in green, pregnenolone in pink. Carbon atoms of the protein are depicted in grey,
the haeme prosthetic group is coloured in blue. Nitrogen, oxygen and sulphur atoms are depicted in blue, red and yellow, respectively.
Relevant water molecules are represented by red points
effects associated with the steroidal scaffold and two under clinical development [40] . Similar to galeterone,
such compounds, orteronel (4) and seviteronel (5), seviteronel works downstream of abiraterone to
have been developed and are subject to clinical trials. inhibit CYP17A1 lyase and does not cause the same
Although, orteronel (TAK-700), an oralimidazole based degree of mineralocorticoid production. This agent
inhibitor [39] had a 5-fold selectivity for 17,20-lyase can therefore be administered without concomitant
activity in comparison with the 17α-hydroxylase activity glucocorticoid administration, resulting in lack of
of CYP17A1, it failed to increase overall survival in associated toxicities (such as muscle wasting, skin
CRPC patients (NCT01193257). friability, cushingoid features, and decreased bone
mineral density).
Seviteronel (VT-464), anorally administered
nonsteroidal CYP17A1 lyase inhibitor, is at the present Recently, Larsen and collaborators identified two
334 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017