Page 62 - Read Online
P. 62
Pippione et al. Steroidogenic enzymes in prostate cancer
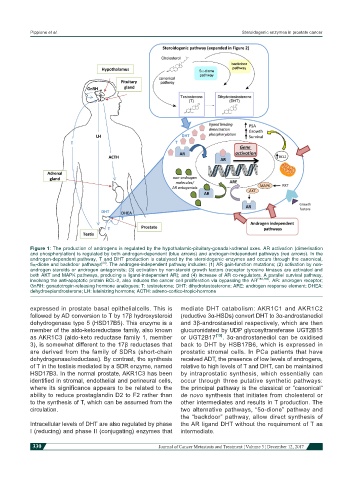
Figure 1: The production of androgens is regulated by the hypothalamic-pituitary-gonadal-adrenal axes. AR activation (dimerisation
and phosphorylation) is regulated by both androgen-dependent (blue arrows) and androgen-independent pathways (red arrows). In the
androgen-dependent pathway, T and DHT production is catalysed by the steroidogenic enzymes and occurs through the canonical,
[24]
5a-dione and backdoor pathways . The androgen-independent pathway includes: (1) AR gain-function mutations; (2) activation by non-
androgen steroids or androgen antagonists; (3) activation by non-steroid growth factors (receptor tyrosine kinases are activated and
both AKT and MAPK pathways, producing a ligand-independent AR); and (4) increase of AR co-regulators. A parallel survival pathway,
involving the anti-apoptotic protein BCL-2, also induces the cancer cell proliferation via bypassing the AR [183,184] . AR: androgen receptor;
GnRH: gonadotropin-releasing hormone analogues; T: testosterone; DHT: dihydrotestosterone; ARE: androgen response element; DHEA:
dehydroepiandrosterone; LH: luteinizing hormone; ACTH: adreno-cortico-tropic-hormone
expressed in prostate basal epithelialcells. This is mediate DHT catabolism: AKR1C1 and AKR1C2
followed by AD conversion to T by 17β hydroxysteroid (reductive 3α-HSDs) convert DHT to 3α-androstanediol
dehydrogenase type 5 (HSD17B5). This enzyme is a and 3β-androstanediol respectively, which are then
member of the aldo-ketoreductase family, also known glucuronidated by UDP glycosyltransferase UGT2B15
as AKR1C3 (aldo-keto reductase family 1, member or UGT2B17 [13] . 3α-androstanediol can be oxidised
3), is somewhat different to the 17β reductases that back to DHT by HSB17B6, which is expressed in
are derived from the family of SDRs (short-chain prostatic stromal cells. In PCa patients that have
dehydrogenase/reductase). By contrast, the synthesis received ADT, the presence of low levels of androgens,
of T in the testisis mediated by a SDR enzyme, named relative to high levels of T and DHT, can be maintained
HSD17B3. In the normal prostate, AKR1C3 has been by intraprostatic synthesis, which essentially can
identified in stromal, endothelial and perineural cells, occur through three putative synthetic pathways:
where its significance appears to be related to the the principal pathway is the classical or “canonical”
ability to reduce prostaglandin D2 to F2 rather than de novo synthesis that initiates from cholesterol or
to the synthesis of T, which can be assumed from the other intermediates and results in T production. The
circulation. two alternative pathways, “5α-dione” pathway and
the “backdoor” pathway, allow direct synthesis of
Intracellular levels of DHT are also regulated by phase the AR ligand DHT without the requirement of T as
I (reducing) and phase II (conjugating) enzymes that intermediate.
330 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017