Page 103 - Read Online
P. 103
Falconer et al. MT-MMPs in prostate cancer
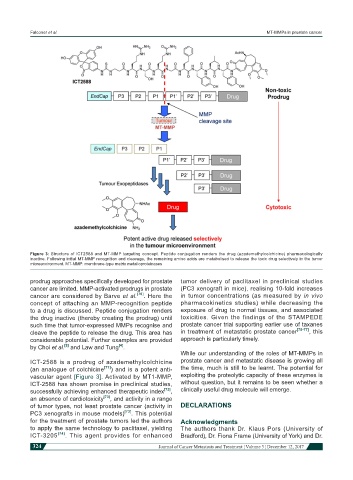
Figure 3: Structure of ICT2588 and MT-MMP targeting concept. Peptide conjugation renders the drug (azademethylcolchicine) pharmacologically
inactive. Following initial MT-MMP recognition and cleavage, the remaining amino acids are metabolised to release the toxic drug selectively in the tumor
microenvironment. MT-MMP: membrane-type matrix metalloproteinases
prodrug approaches specifically developed for prostate tumor delivery of paclitaxel in preclinical studies
cancer are limited. MMP-activated prodrugs in prostate (PC3 xenograft in mice), realising 10-fold increases
cancer are considered by Barve et al. [70] . Here the in tumor concentrations (as measured by in vivo
concept of attaching an MMP-recognition peptide pharmacokinetics studies) while decreasing the
to a drug is discussed. Peptide conjugation renders exposure of drug to normal tissues, and associated
the drug inactive (thereby creating the prodrug) until toxicities. Given the findings of the STAMPEDE
such time that tumor-expressed MMPs recognise and prostate cancer trial supporting earlier use of taxanes
cleave the peptide to release the drug. This area has in treatment of metastatic prostate cancer [75-77] , this
considerable potential. Further examples are provided approach is particularly timely.
[4]
[3]
by Choi et al. and Law and Tung .
While our understanding of the roles of MT-MMPs in
ICT-2588 is a prodrug of azademethylcolchicine prostate cancer and metastatic disease is growing all
(an analogue of colchicine [71] ) and is a potent anti- the time, much is still to be learnt. The potential for
vascular agent [Figure 3]. Activated by MT1-MMP, exploiting the proteolytic capacity of these enzymes is
ICT-2588 has shown promise in preclinical studies, without question, but it remains to be seen whether a
successfully achieving enhanced therapeutic index [72] , clinically useful drug molecule will emerge.
an absence of cardiotoxicity [73] , and activity in a range
of tumor types, not least prostate cancer (activity in DECLARATIONS
PC3 xenografts in mouse models) [73] . This potential
for the treatment of prostate tumors led the authors Acknowledgments
to apply the same technology to paclitaxel, yielding The authors thank Dr. Klaus Pors (University of
ICT-3205 [74] . This agent provides for enhanced Bradford), Dr. Fiona Frame (University of York) and Dr.
324 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017