Page 43 - Read Online
P. 43
Terai et al. The liver and metastatic uveal melanoma
A B
C D
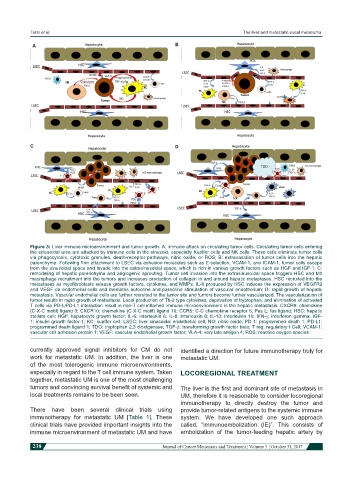
Figure 3: Liver immune microenvironment and tumor growth. A: immune attack on circulating tumor cells. Circulating tumor cells entering
the sinusoidal area are attacked by immune cells in the sinusoid, especially Kupffer cells and NK cells. These cells eliminate tumor cells
via phagocytosis, cytotoxic granules, death-receptor pathways, nitric oxide, or ROS; B: extravasation of tumor cells into the hepatic
parenchyma. Following firm attachment to LSEC via adhesion molecules such as E-selection, VCAM-1, and ICAM-1, tumor cells escape
from the sinusoidal space and invade into the extrasinusoidal space, which is rich in various growth factors such as HGF and IGF-1; C:
remodeling of hepatic parenchyma and angiogenic sprouting. Tumor cell invasion into the extrasinusoidal space triggers HSC and M2
macrophage recruitment into the tumors and increases production of collagen in and around hepatic metastases. HSC recruited into the
metastases as myofibroblasts release growth factors, cytokines, and MMPs. IL-8 produced by HSC induces the expression of VEGFR2
and VEGF on endothelial cells and mediates autocrine and paracrine stimulation of vascular endothelium; D: rapid growth of hepatic
metastasis. Vascular endothelial cells are further recruited to the tumor site and tumors become further vascularized. The vascularization of
tumor results in rapid growth of metastasis. Local production of Th-2 type cytokines, deprivation of tryptophan, and elimination of activated
T cells via PD-L/PD-L1 interaction result in non-T cell inflamed immune microenvironment in the hepatic metastasis. CXCR9: chemokine
(C-X-C motif) ligand 9; CXCR10: chemokine (C-X-C motif) ligand 10; CCR5: C-C chemokine receptor 5; Fas L: fas ligand; HSC: hepatic
stellate cell; HGF: hepatocyte growth factor; IL-6: interleukin 6; IL-8: interleukin 8; IL-10: interleukin 10; IFN-g: interferon gamma; IGF-
1: insulin growth factor-1; KC: kupffer cell; LSEC: liver sinusoidal endothelial cell; NO: nitric oxide; PD 1: programmed death 1; PD-L1:
programmed death ligand 1; TDO: tryptophan 2,3 dioxtgenase; TGF-b: transforming growth factor beta; T reg: regulatory t Cell; VCAM-1:
vascular cell adhesion protein 1; VEGF: vascular endothelial growth factor; VLA-4: very late antigen 4; ROS: reactive oxygen species
currently approved signal inhibitors for CM do not identified a direction for future immunotherapy truly for
work for metastatic UM. In addition, the liver is one metastatic UM.
of the most tolerogenic immune microenvironments,
especially in regard to the T cell immune system. Taken LOCOREGIONAL TREATMENT
together, metastatic UM is one of the most challenging
tumors and convincing survival benefit of systemic and The liver is the first and dominant site of metastasis in
local treatments remains to be been seen. UM, therefore it is reasonable to consider locoregional
immunotherapy to directly destroy the tumor and
There have been several clinical trials using provide tumor-related antigens to the systemic immune
immunotherapy for metastatic UM [Table 1]. These system. We have developed one such approach
clinical trials have provided important insights into the called, “immunoembolization (IE)”. This consists of
immune microenvironment of metastatic UM and have embolization of the tumor-feeding hepatic artery by
238 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ October 31, 2017