Page 44 - Read Online
P. 44
Terai et al. The liver and metastatic uveal melanoma
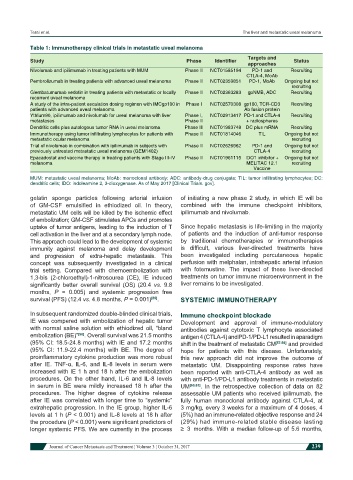
Table 1: Immunotherapy clinical trials in metastatic uveal melanoma
Targets and
Study Phase Identifier Status
approaches
Nivolumab and ipilimumab in treating patients with MUM Phase II NCT01585194 PD-1 and Recruiting
CTLA-4, MoAb
Pembrolizumab in treating patients with advanced uveal melanoma Phase II NCT02359851 PD-1, MoAb Ongoing but not
recruiting
Glembatumumab vedotin in treating patients with metastatic or locally Phase II NCT02363283 gpNMB, ADC Recruiting
recurrent uveal melanoma
A study of the intra-patient escalation dosing regimen with IMCgp100 in Phase I NCT02570308 gp100, TCR-CD3 Recruiting
patients with advanced uveal melanoma Ab fusion protein
Yttrium90, ipilimumab and nivolumab for uveal melanoma with liver Phase I, NCT02913417 PD-1 and CTLA-4 Recruiting
metastases Phase II + radiospheres
Dendritic cells plus autologous tumor RNA in uveal melanoma Phase III NCT01983748 DC plus mRNA Recruiting
Immunotherapy using tumor infiltrating lymphocytes for patients with Phase II NCT01814046 TIL Ongoing but not
metastatic ocular melanoma recruiting
Trial of nivolumab in combination with ipilimumab in subjects with Phase II NCT02626962 PD-1 and Ongoing but not
previously untreated metastatic uveal melanoma (GEM1402) CTLA-4 recruiting
Epacadostat and vaccine therapy in treating patients with Stage III-IV Phase II NCT01961115 IDO1 inhibitor + Ongoing but not
melanoma MELITAC 12.1 recruiting
Vaccine
MUM: metastatic uveal melanoma; MoAb: monoclonal antibody; ADC: antibody-drug conjugate; TIL: tumor infiltrating lymphocytes; DC:
dendritic cells; IDO: indoleamine 2, 3-dioxygenase. As of May 2017 [Clinical Trials. gov].
gelatin sponge particles following arterial infusion of initiating a new phase 2 study, in which IE will be
of GM-CSF emulsified in ethiodized oil. In theory, combined with the immune checkpoint inhibitors,
metastatic UM cells will be killed by the ischemic effect ipilimumab and nivolumab.
of embolization; GM-CSF stimulates APCs and promotes
uptake of tumor antigens, leading to the induction of T Since hepatic metastasis is life-limiting in the majority
cell activation in the liver and at a secondary lymph node. of patients and the induction of anti-tumor response
This approach could lead to the development of systemic by traditional chemotherapies or immunotherapies
immunity against melanoma and delay development is difficult, various liver-directed treatments have
and progression of extra-hepatic metastasis. This been investigated including percutaneous hepatic
concept was subsequently investigated in a clinical perfusion with melphalan, intrahepatic arterial infusion
trial setting. Compared with chemoembolization with with fotemustine. The impact of these liver-directed
1,3-bis (2-chloroethyl)-1-nitrosourea (CE), IE induced treatments on tumor immune microenvironment in the
significantly better overall survival (OS) (20.4 vs. 9.8 liver remains to be investigated.
months, P = 0.005) and systemic progression free
survival (PFS) (12.4 vs. 4.8 months, P = 0.001) [55] . SYSTEMIC IMMUNOTHERAPY
In subsequent randomized double-blinded clinical trials, Immune checkpoint blockade
IE was compered with embolization of hepatic tumor Development and approval of immune-modulatory
with normal saline solution with ethiodized oil, “bland antibodies against cytotoxic T lymphocyte associated
embolization (BE)” [56] . Overall survival was 21.5 months antigen 4 (CTLA-4) and PD-1/PD-L1 resulted in aparadigm
(95% CI: 18.5-24.8 months) with IE and 17.2 months shift in the treatment of metastatic CM [57-59] and provided
(95% CI: 11.9-22.4 months) with BE. The degree of hope for patients with this disease. Unfortunately,
proinflammatory cytokine production was more robust this new approach did not improve the outcome of
after IE. TNF-α, IL-6, and IL-8 levels in serum were metastatic UM. Disappointing response rates have
increased with IE 1 h and 18 h after the embolization been reported with anti-CTLA-4 antibody as well as
procedures. On the other hand, IL-6 and IL-8 levels with anti-PD-1/PD-L1 antibody treatments in metastatic
in serum in BE were mildly increased 18 h after the UM [60,61] . In the retrospective collection of data on 82
procedures. The higher degree of cytokine release assessable UM patients who received ipilimumab, the
after IE was correlated with longer time to “systemic” fully human monoclonal antibody against CTLA-4, at
extrahepatic progression. In the IE group, higher IL-6 3 mg/kg, every 3 weeks for a maximum of 4 doses, 4
levels at 1 h (P < 0.001) and IL-8 levels at 18 h after (5%) had an immune-related objective response and 24
the procedure (P < 0.001) were significant predictors of (29%) had immune-related stable disease lasting
longer systemic PFS. We are currently in the process ≥ 3 months. With a median follow-up of 5.6 months,
Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ October 31, 2017 239