Page 942 - Read Online
P. 942
Caron de Fromentel et al. Hepatoma Res 2020;6:80 I http://dx.doi.org/10.20517/2394-5079.2020.77 Page 11 of 18
A B
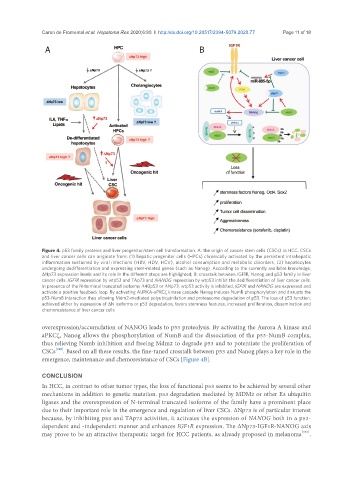
Figure 4. p53 family proteins and liver progenitor/stem cell transformation. A: the origin of cancer stem cells (CSCs) in HCC. CSCs
and liver cancer cells can originate from: (1) hepatic progenitor cells (HPCs) chronically activated by the persistent intrahepatic
inflammation sustained by viral infections (HBV, HDV, HCV), alcohol consumption and metabolic disorders; (2) hepatocytes
undergoing dedifferentiation and expressing stem-related genes (such as Nanog). According to the currently available knowledge,
ΔNp73 expression levels and its role in the different steps are highlighted; B: crosstalk between IGF1R, Nanog and p53 family in liver
cancer cells. IGF1R repression by wtp53 and TAp73 and NANOG repression by wtp53 inhibit the dedifferentiation of liver cancer cells.
In presence of the N-terminal truncated isoforms Δ40p53 or ΔNp73, wtp53 activity is inhibited, IGF1R and NANOG are expressed and
activate a positive feedback loop. By activating AURKA-aPKCz kinase cascade Nanog induces NumB phosphorylation and disrupts the
p53-NumB interaction thus allowing Mdm2-mediated polyubiquinilation and proteasome degradation of p53. The loss of p53 function,
achieved either by expression of ΔN isoforms or p53 degradation, favors stemness features, increased proliferation, dissemination and
chemoresistance of liver cancer cells
overexpression/accumulation of NANOG leads to p53 proteolysis. By activating the Aurora A kinase and
aPKCz, Nanog allows the phosphorylation of NumB and the dissociation of the p53-NumB complex,
thus relieving Numb inhibition and freeing Mdm2 to degrade p53 and to potentiate the proliferation of
CSCs [180] . Based on all these results, the fine-tuned crosstalk between p53 and Nanog plays a key role in the
emergence, maintenance and chemoresistance of CSCs [Figure 4B].
CONCLUSION
In HCC, in contrast to other tumor types, the loss of functional p53 seems to be achieved by several other
mechanisms in addition to genetic mutation. p53 degradation mediated by MDM2 or other E3 ubiquitin
ligases and the overexpression of N-terminal truncated isoforms of the family have a prominent place
due to their important role in the emergence and regulation of liver CSCs. ΔNp73 is of particular interest
because, by inhibiting p53 and TAp73 activities, it activates the expression of NANOG both in a p53-
dependent and -independent manner and enhances IGF1R expression. The ΔNp73-IGF1R-NANOG axis
may prove to be an attractive therapeutic target for HCC patients, as already proposed in melanoma [134] .