Page 859 - Read Online
P. 859
Page 10 of 16 Silk et al. Hepatoma Res 2020;6:73 I http://dx.doi.org/10.20517/2394-5079.2020.61
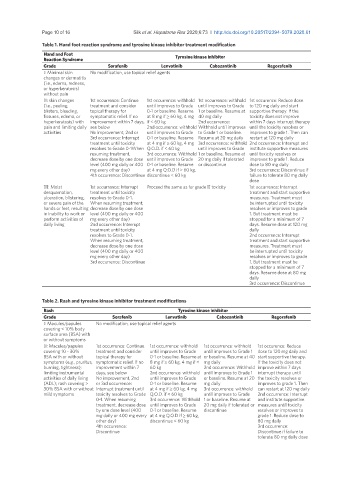
Table 1. Hand foot reaction syndrome and tyrosine kinase inhibitor treatment modification
Hand and Foot
Reaction Syndrome Tyrosine kinase inhibitor
Grade Sorafenib Lenvatinib Cabozantinib Regorafenib
I: Minimal skin No modification, use topical relief agents
changes or dermatitis
(i.e., edema, redness,
or hyperkeratosis)
without pain
II: skin changes 1st occurrence: Continue 1st occurrence: withhold 1st occurrence: withhold 1st occurence: Reduce dose
(i.e., peeling, treatment and consider until improves to Grade until improves to Grade to 120 mg daily and start
blisters, bleeding, topical therapy for 0-1 or baseline. Resume 1 or baseline. Resume at supportive therapy. If the
fissures, edema, or symptomatic relief. If no at 8 mg if ≥ 60 kg, 4 mg 40 mg daily toxicity does not improve
hyperkeratosis) with improvement within 7 days, if < 60 kg 2nd occurrence: within 7 days interrupt therapy
pain and limiting daily see below 2nd occurrence: withhold Withhold until improves until the toxicity resolves or
activities No improvement, 2nd or until improves to Grade to Grade 1 or baseline. improves to grade 1. Then can
3rd occurrence: Interrupt 0-1 or baseline. Resume Resume at 20 mg daily restart at 120 mg daily
treatment until toxicity at 4 mg if ≥ 60 kg, 4 mg 3rd occurrence: withhold 2nd occurrence: Interrupt and
resolves to Grade 0-1When Q.O.D. if < 60 kg until improves to Grade institute supportive measures
resuming treatment, 3rd occurrence: Withhold 1 or baseline. Resume at until toxicity resolves or
decrease dose by one dose until improves to Grade 20 mg daily if tolerated improves to grade 1. Reduce
level (400 mg daily or 400 0-1 or baseline. Resume or discontinue dose to 80 mg daily
mg every other day) at 4 mg Q.O.D if ≥ 60 kg, 3rd occurrence: Discontinue if
4th occurrence: Discontinue discontinue < 60 kg failure to tolerate 80 mg daily
dose
III: Moist 1st occurrence: Interrupt Proceed the same as for grade II toxicity 1st occurrence: Interrupt
desquamation, treatment until toxicity treatment and start supportive
ulceration, blistering, resolves to Grade 0-1. measures. Treatment must
or severe pain of the When resuming treatment, be interrupted until toxicity
hands or feet, resulting decrease dose by one dose resolves or improves to grade
in inability to work or level (400 mg daily or 400 1. But treatment must be
perform activities of mg every other day) stopped for a minimum of 7
daily living 2nd occurrence: Interrupt days. Resume dose at 120 mg
treatment until toxicity daily
resolves to Grade 0-1. 2nd occurrence: Interrupt
When resuming treatment, treatment and start supportive
decrease dose by one dose measures. Treatment must
level (400 mg daily or 400 be interrupted until toxicity
mg every other day) resolves or improves to grade
3rd occurrence: Discontinue 1. But treatment must be
stopped for a minimum of 7
days. Resume dose at 80 mg
daily
3rd occurrence: Discontinue
Table 2. Rash and tyrosine kinase inhibitor treatment modifications
Rash Tyrosine kinase inhibitor
Grade Sorafenib Lenvatinib Cabozantinib Regorafenib
I: Macules/papules No modification, use topical relief agents
covering < 10% body
surface area (BSA) with
or without symptoms
II: Macules/papules 1st occurrence: Continue 1st occurrence: withhold 1st occurrence: withhold 1st occurence: Reduce
covering 10 - 30% treatment and consider until improves to Grade until improves to Grade 1 dose to 120 mg daily and
BSA with or without topical therapy for 0-1 or baseline. Resume at or baseline. Resume at 40 start supportive therapy.
symptoms (e.g., pruritus, symptomatic relief. If no 8 mg if ≥ 60 kg, 4 mg if < mg daily If the toxicity does not
burning, tightness); improvement within 7 60 kg 2nd occurrence: Withhold improve within 7 days
limiting instrumental days, see below 2nd occurrence: withhold until improves to Grade 1 interrupt therapy until
activities of daily living No improvement, 2nd until improves to Grade or baseline. Resume at 20 the toxicity resolves or
(ADL); rash covering > or 3rd occurrence: 0-1 or baseline. Resume mg daily improves to grade 1. Then
30% BSA with or without Interrupt treatment until at 4 mg if ≥ 60 kg, 4 mg 3rd occurrence: withhold can restart at 120 mg daily
mild symptoms toxicity resolves to Grade Q.O.D. if < 60 kg until improves to Grade 2nd occurrence: Interrupt
0-1. When resuming 3rd occurrence: Withhold 1 or baseline. Resume at and institute supportive
treatment, decrease dose until improves to Grade 20 mg daily if tolerated or measures until toxicity
by one dose level (400 0-1 or baseline. Resume discontinue resolves or improves to
mg daily or 400 mg every at 4 mg Q.O.D if ≥ 60 kg, grade 1. Reduce dose to
other day) discontinue < 60 kg 80 mg daily
4th occurrence: 3rd occurrence:
Discontinue Discontinue if failure to
tolerate 80 mg daily dose