Page 769 - Read Online
P. 769
Shimizuguchi et al. Hepatoma Res 2020;6:66 I http://dx.doi.org/10.20517/2394-5079.2020.51 Page 7 of 10
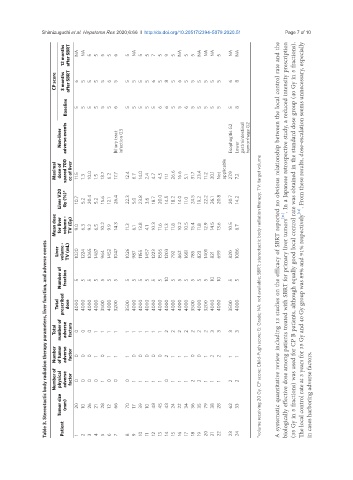
12 months after SBRT NA NA 5 5 5 5 6 5 NA 5 5 7 5 9 5 NA 5 5 NA NA NA 5 NA NA
CP score 3 months after SBRT 6 5 5 5 5 6 5 5 5 5 5 6 5 8 5 6 5 5 5 5 5 5 6 8
Baseline 5 5 5 5 5 5 6 5 5 5 5 5 6 6 5 5 6 5 5 5 5 5 5 8
Non-liver adverse events Biliary tract infection G3 Esophagitis G2 Lower gastrointestinal hemorrhage G2
Maximal dose of spared 700 cc of liver 11.5 1.3 10.0 1.5 13.7 6.2 17.7 12.4 6.7 14.0 2.4 4.7 4.5 11.1 26.6 16.6 5.1 31.7 23.4 11.2 30.1 Not applicable 27.9 7.2
Liver V20 Gy (%)* 10.7 5.2 20.4 5.2 15.6 12.1 26.4 22.3 5.0 23.8 3.6 18.7 20.0 14.5 18.2 14.0 11.0 24.5 19.2 22.2 26.1 20.8 20.7 14.2
Mean dose for liver volume - TV (Gy) 9.0 6.3 9.2 6.5 10.2 9.9 14.3 11.3 6.1 13.8 4.1 10.3 11.6 11.3 11.8 10.2 10.5 15.4 11.8 12.9 14.5 13.6 10.5 8.7
Table 3. Stereotactic body radiation therapy parameters, liver function, and adverse events
Liver volume - TV (mL) 1020 1224 1065 1487 964 1452 1047 1026 987 1155 1457 1223 1556 1203 792 862 1681 785 823 1401 821 699 820 1086 A systematic quantitative review including 13 studies on the efficacy of SBRT reported no obvious relationship between the local control rate and the biologically effective dose among patients treated with SBRT for primary liver tumors [11] . In a Japanese retrospective study, a reduced inte
Number of fraction 5 5 5 5 5 5 5 5 5 5 5 5 5 10 5 5 5 5 5 5 10 10 5 5
Total prescribed dose 4000 4000 4000 4000 3500 4000 3200 3500 4000 4000 4000 4000 4000 4000 4000 4000 4000 3500 4000 3200 4000 4000 3500 4000 *volume receiving 20 Gy. CP score: Child-Pugh score; G: Grade; NA: not available; SBRT: stereotactic body radiation therapy; TV: target volume
Total number of adverse factors 0 0 0 1 1 1 1 1 1 1 1 1 1 2 2 2 2 2 2 2 3 3 3 3
Number of tumor adverse factor 0 0 0 1 0 1 1 1 0 0 0 0 0 2 1 1 1 0 0 1 2 2 1 1
Number of physical adverse factor 0 0 0 0 1 0 0 0 1 1 1 1 1 0 1 1 1 2 2 1 1 1 2 2
Tumor size (mm) 20 10 26 21 28 12 66 70 17 39 10 48 45 43 24 22 34 36 35 79 38 28 62 33 in cases harboring adverse factors.
Patient 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24