Page 766 - Read Online
P. 766
Page 4 of 10 Shimizuguchi et al. Hepatoma Res 2020;6:66 I http://dx.doi.org/10.20517/2394-5079.2020.51
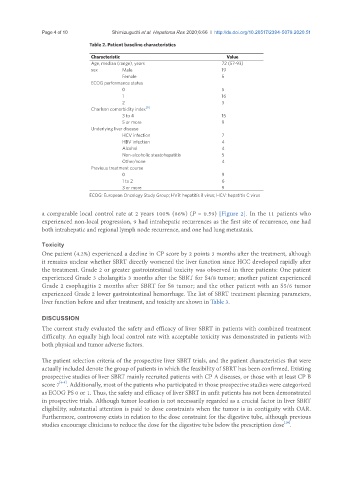
Table 2. Patient baseline characteristics
Characteristic Value
Age, median (range), years 72 (57-93)
sex Male 19
Female 5
ECOG performance status
0 5
1 16
2 3
Charlson comorbidity index [9]
3 to 4 15
5 or more 9
Underlying liver disease
HCV infection 7
HBV infection 4
Alcohol 4
Non-alcoholic steatohepatitis 5
Other/none 4
Previous treatment course
0 9
1 to 2 6
3 or more 9
ECOG: European Oncology Study Group; HVB: hepatitis B virus; HCV: hepatitis C virus
a comparable local control rate at 2 years 100% (86%) (P = 0.59) [Figure 2]. In the 11 patients who
experienced non-local progression, 9 had intrahepatic recurrences as the first site of recurrence, one had
both intrahepatic and regional lymph node recurrence, and one had lung metastasis.
Toxicity
One patient (4.2%) experienced a decline in CP score by 2 points 3 months after the treatment, although
it remains unclear whether SBRT directly worsened the liver function since HCC developed rapidly after
the treatment. Grade 2 or greater gastrointestinal toxicity was observed in three patients: One patient
experienced Grade 3 cholangitis 3 months after the SBRT for S4/8 tumor; another patient experienced
Grade 2 esophagitis 2 months after SBRT for S8 tumor; and the other patient with an S5/6 tumor
experienced Grade 2 lower gastrointestinal hemorrhage. The list of SBRT treatment planning parameters,
liver function before and after treatment, and toxicity are shown in Table 3.
DISCUSSION
The current study evaluated the safety and efficacy of liver SBRT in patients with combined treatment
difficulty. An equally high local control rate with acceptable toxicity was demonstrated in patients with
both physical and tumor adverse factors.
The patient selection criteria of the prospective liver SBRT trials, and the patient characteristics that were
actually included denote the group of patients in which the feasibility of SBRT has been confirmed. Existing
prospective studies of liver SBRT mainly recruited patients with CP A diseases, or those with at least CP B
[6-8]
score 7 . Additionally, most of the patients who participated in those prospective studies were categorized
as ECOG PS 0 or 1. Thus, the safety and efficacy of liver SBRT in unfit patients has not been demonstrated
in prospective trials. Although tumor location is not necessarily regarded as a crucial factor in liver SBRT
eligibility, substantial attention is paid to dose constraints when the tumor is in contiguity with OAR.
Furthermore, controversy exists in relation to the dose constraint for the digestive tube, although previous
[10]
studies encourage clinicians to reduce the dose for the digestive tube below the prescription dose .