Page 123 - Read Online
P. 123
Gomaa et al. Advanced HCC: current and potential therapies
Second-line therapy
10.6
9.4 9.2
8.2 7.6 7.3 7.6 7.8
Overall survival (months)
Brivanib Placebo Everolimus Placebo Ramucirumab Placebo Regorafenib Placebo
[33]
[32]
[29]
[34]
Llovet et al., 2013 Zhu et al., 2014 Zhu et al., 2015 Bruix et al., 2017
PRISK-PS EVOLVE-1 REACH RESORSE
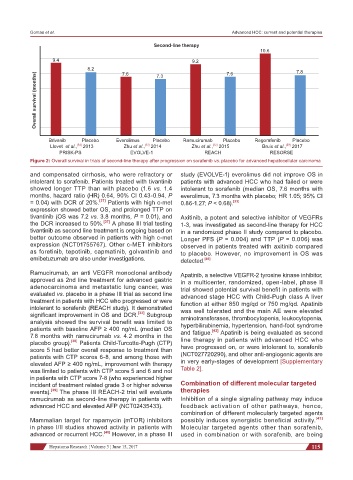
Figure 2: Overall survival in trials of second-line therapy after progression on sorafenib vs. placebo for advanced hepatocellular carcinoma
and compensated cirrhosis, who were refractory or study (EVOLVE-1) everolimus did not improve OS in
intolerant to sorafenib. Patients treated with tivantinib patients with advanced HCC who had failed or were
showed longer TTP than with placebo (1.6 vs. 1.4 intolerant to sorafenib (median OS, 7.6 months with
months, hazard ratio (HR) 0.64, 90% CI 0.43-0.94, P everolimus, 7.3 months with placebo; HR 1.05; 95% CI
= 0.04) with DCR of 20%. [37] Patients with high c-met 0.86-1.27; P < 0.68). [33]
expression showed better OS, and prolonged TTP on
tivantinib (OS was 7.2 vs. 3.8 months, P = 0.01), and Axitinib, a potent and selective inhibitor of VEGFRs
the DCR increased to 50%. [37] A phase III trial testing 1-3, was investigated as second-line therapy for HCC
tivantinib as second line treatment is ongoing based on in a randomized phase II study compared to placebo.
better outcome observed in patients with high c-met Longer PFS (P = 0.004) and TTP (P = 0.006) was
expression (NCT01755767). Other c-MET inhibitors observed in patients treated with axitinib compared
as foretinib, tepotinib, capmatinib, golvantinib and to placebo. However, no improvement in OS was
emibetuzumab are also under investigations. detected. [41]
Ramucirumab, an anti VEGFR monoclonal antibody Apatinib, a selective VEGFR-2 tyrosine kinase inhibitor,
approved as 2nd line treatment for advanced gastric in a multicenter, randomized, open-label, phase II
adenocarcinoma and metastatic lung cancer, was trial showed potential survival benefit in patients with
evaluated vs. placebo in a phase III trial as second line advanced stage HCC with Child-Pugh class A liver
treatment in patients with HCC who progressed or were function at either 850 mg/qd or 750 mg/qd. Apatinib
intolerant to sorafenib (REACH study). It demonstrated was well tolerated and the main AE were elevated
significant improvement in OS and DCR. [32] Subgroup aminotransferases, thrombocytopenia, leukocytopenia,
analysis showed the survival benefit was limited to hyperbilirubinemia, hypertension, hand-foot syndrome
patients with baseline AFP ≥ 400 ng/mL (median OS and fatigue. [42] Apatinib is being evaluated as second
7.8 months with ramucirumab vs. 4.2 months in the line therapy in patients with advanced HCC who
placebo group). [38] Patients Child-Turcotte-Pugh (CTP)
score 5 had better overall response to treatment than have progressed on, or were intolerant to, sorafenib
patients with CTP scores 6-8, and among those with (NCT027720290), and other anti-angiogenic agents are
elevated AFP ≥ 400 ng/mL, improvement with therapy in very early-stages of development [Supplementary
was limited to patients with CTP score 5 and 6 and not Table 2].
in patients with CTP score 7-8 (who experienced higher
incident of treatment related grade 3 or higher adverse Combination of different molecular targeted
events). [39] The phase III REACH-2 trial will evaluate therapies
ramucirumab as second-line therapy in patients with Inhibition of a single signaling pathway may induce
advanced HCC and elevated AFP (NCT02435433). feedback activation of other pathways, hence,
combination of different molecularly targeted agents
Mammalian target for rapamycin (mTOR) inhibitors possibly induces synergistic beneficial activity. [43]
in phase I/II studies showed activity in patients with Molecular targeted agents other than sorafenib,
advanced or recurrent HCC. [40] However, in a phase III used in combination or with sorafenib, are being
Hepatoma Research ¦ Volume 3 ¦ June 15, 2017 115