Page 33 - Read Online
P. 33
a b
a b
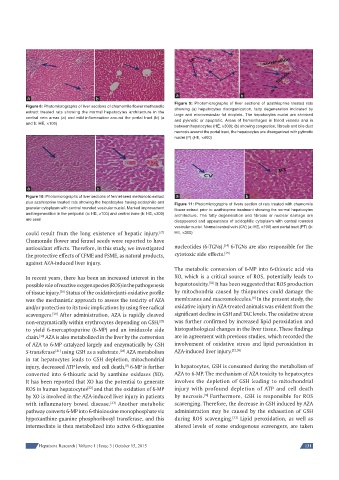
Figure 9: Photomicrographs of liver sections of azathioprine treated rats
Figure 8: Photomicrographs of liver sections of chamomile fl ower methanolic showing (a) hepatocytes disorganization, fatty degeneration indicated by
extract treated rats showing the normal hepatocytes architecture in the large and microvesicular fat droplets. The hepatocytes nuclei are shrinked
central vein areas (a) and mild infl ammation around the portal tract (b) (a and pyknotic or apoptotic. Areas of hemorrhages in blood vessels and in
and b: HE, ×100)
between hepatocytes (HE, ×300); (b) showing congestion, fi brosis and bile duct
necrosis around the portal tract, the hepatocytes are disorganized with pyknotic
nuclei (P) (HE, ×400)
a b
Figure 10: Photomicrographs of liver sections of fennel seed methanolic extract a b
plus azathioprine treated rats showing the hepatocytes having acidophilic and Figure 11: Photomicrographs of livers section of rats treated with chamomile
granular cytoplasm with central rounded vesicular nuclei. Marked improvement fl ower extract prior to azathioprine treatment showing the normal hepatocytes
and regeneration in the periportal (a: HE, ×100) and central zone (b: HE, ×300) architecture. The fatty degeneration and fibrosis or nuclear damage are
are seen disappeared and appearance of acidophilic cytoplasm with central rounded
vesicular nuclei. Normal central vein (CV) (a: HE, ×100) and portal tract (PT) (b:
could result from the long existence of hepatic injury. HE, ×300)
[27]
Chamomile flower and fennel seeds were reported to have
[34]
antioxidant effects. Therefore, in this study, we investigated nucleotides (6-TGNs). 6-TGNs are also responsible for the
the protective effects of CFME and FSME, as natural products, cytotoxic side effects. [35]
against AZA-induced liver injury.
The metabolic conversion of 6-MP into 6-thiouric acid via
In recent years, there has been an increased interest in the XO, which is a critical source of ROS, potentially leads to
[32]
possible role of reactive oxygen species (ROS) in the pathogenesis hepatotoxicity. It has been suggested that ROS production
of tissue injury. Status of the oxidative/anti-oxidative profile by mitochondria caused by thiopurines could damage the
[26]
[8]
was the mechanistic approach to assess the toxicity of AZA membranes and macromolecules. In the present study, the
and/or protection to its toxic implications by using free radical oxidative injury in AZA-treated animals was evident from the
scavengers. After administration, AZA is rapidly cleaved significant decline in GSH and TAC levels. The oxidative stress
[28]
non-enzymatically within erythrocytes depending on GSH, was further confirmed by increased lipid peroxidation and
[29]
to yield 6-mercaptopurine (6-MP) and an imidazole side histopathological changes in the liver tissue. These findings
chain. AZA is also metabolized in the liver by the conversion are in agreement with previous studies, which recorded the
[30]
of AZA to 6-MP catalyzed largely and enzymatically by GSH involvement of oxidative stress and lipid peroxidation in
S-transferase using GSH as a substrate. AZA metabolism AZA-induced liver injury. [32,36]
[28]
[31]
in rat hepatocytes leads to GSH depletion, mitochondrial
injury, decreased ATP levels, and cell death. 6-MP is further In hepatocytes, GSH is consumed during the metabolism of
[8]
converted into 6-thiouric acid by xanthine oxidases (XO). AZA to 6-MP. The mechanism of AZA toxicity to hepatocytes
It has been reported that XO has the potential to generate involves the depletion of GSH leading to mitochondrial
ROS in human hepatocytes and that the oxidation of 6-MP injury with profound depletion of ATP and cell death
[32]
[9]
by XO is involved in the AZA-induced liver injury in patients by necrosis. Furthermore, GSH is responsible for ROS
with inflammatory bowel disease. Another metabolic scavenging. Therefore, the decrease in GSH induced by AZA
[33]
pathway converts 6-MP into 6-thioinosine monophosphate via administration may be caused by the exhaustion of GSH
hypoxanthine-guanine phosphoribosyl transferase, and this during ROS scavenging. Lipid peroxidation, as well as
[32]
intermediate is then metabolized into active 6-thioguanine altered levels of some endogenous scavengers, are taken
Hepatoma Research | Volume 1 | Issue 3 | October 15, 2015 131