Page 63 - Read Online
P. 63
Page 6 of 23 Casolino et al. Hepatoma Res 2021;7:76 https://dx.doi.org/10.20517/2394-5079.2021.79
Table 2. Cytotoxic chemotherapy: selection of ongoing clinical trials
Trial number Phase Approach Drug(s) Line of treatment
NCT04163900 3 Unselected NUC-1031 + cisplatin I
NCT03768414 3 Unselected Gemcitabine hydrochloride + cisplatin + nab-Paclitaxel I
NCT04066491 2/3 Unselected GEM/CIS + bintrafusp alfa (M7824) I
NCT04005339 2 Unselected Fluorouracil + leucovorin + NALIRI II
NCT03044587 2 Unselected Fluorouracil + leucovorin + NALIRI I
NCT03043547 2 Unselected NALIRI + 5-FU II
NCT04111380 2 Unselected Nab-paclitaxel + cisplatin II
NCT04692051 2 Unselected Nab-paclitaxel + cisplatin I
NCT04203160 2 Unselected GEM/CIS + devimistat (CPI-613) I
NCT04076761 2 Unselected Trifluridine/tipiracil II
NCT03943043 1/2 Unselected GEMOX + Nab-paclitaxel II
NCT03368963 1/2 Unselected Trifluridine/tipiracil + NALIRI II
NCT04491942 1 Unselected Cisplatin or GEM/CIS + elimusertib (BAY 1895344) II
GEMOX: Gemcitabine + oxaliplatin; GEM/CIS: gemcitabine + cisplatin; FOLFOX: 5-fluorouracil + folinic acid + oxaliplatin; NALIRI: nanoliposomal
irinotecan; 5-FU: 5-fluorouracil.
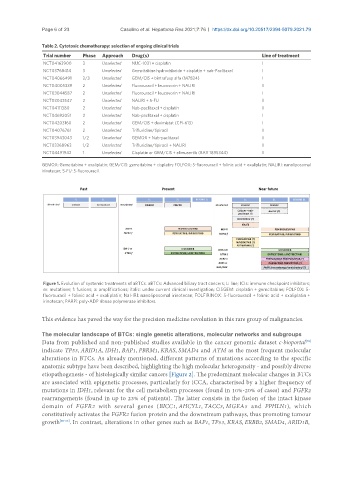
Figure 1. Evolution of systemic treatments of aBTCs. aBTCs: Advanced biliary tract cancers; L: line; ICIs: immune checkpoint inhibitors;
m: mutations; f: fusions; a: amplifications; italic: under current clinical investigation; CISGEM: cisplatin + gemcitabine; FOLFOX: 5-
fluorouracil + folinic acid + oxaliplatin; Nal-IRI: nanoliposomal irinotecan; FOLFIRINOX: 5-fluorouracil + folinic acid + oxaliplatin +
irinotecan; PARPi: poly-ADP ribose polymerase inhibitors.
This evidence has paved the way for the precision medicine revolution in this rare group of malignancies.
The molecular landscape of BTCs: single genetic alterations, molecular networks and subgroups
Data from published and non-published studies available in the cancer genomic dataset c-bioportal
[59]
indicate TP53, ARID1A, IDH1, BAP1, PBRM1, KRAS, SMAD4 and ATM as the most frequent molecular
alterations in BTCs. As already mentioned, different patterns of mutations according to the specific
anatomic subtype have been described, highlighting the high molecular heterogeneity - and possibly diverse
etiopathogenesis - of histologically similar cancers [Figure 2]. The predominant molecular changes in BTCs
are associated with epigenetic processes, particularly for iCCA, characterised by a higher frequency of
mutations in IDH1, relevant for the cell metabolism processes (found in 10%-20% of cases) and FGFR2
rearrangements (found in up to 23% of patients). The latter consists in the fusion of the intact kinase
domain of FGFR2 with several genes (BICC1, AHCYL1, TACC3, MGEA5 and PPHLN1), which
constitutively activates the FGFR2 fusion protein and the downstream pathways, thus promoting tumour
growth [60-66] . In contrast, alterations in other genes such as BAP1, TP53, KRAS, ERBB2, SMAD4, ARID1B,