Page 67 - Read Online
P. 67
Page 10 of 23 Casolino et al. Hepatoma Res 2021;7:76 https://dx.doi.org/10.20517/2394-5079.2021.79
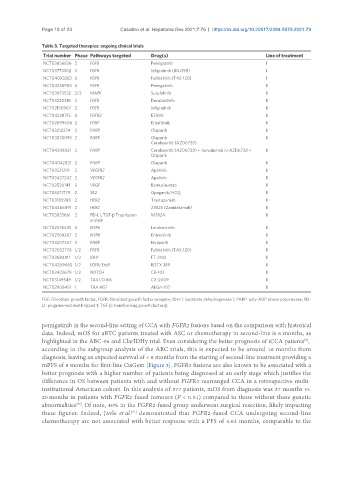
Table 3. Targeted therapies: ongoing clinical trials
Trial number Phase Pathways targeted Drug(s) Line of treatment
NCT03656536 3 FGFR Pemigatinib I
NCT03773302 3 FGFR Infigratinib (BGJ398) I
NCT04093362 3 FGFR Futibatinib (TAS-120) I
NCT04256980 2 FGFR Pemigatinib II
NCT03873532 2/3 MAPK Surufatinib II
NCT03230318 2 FGFR Derazantinib II
NCT02150967 2 FGFR Infigratinib II
NCT04238715 2 FGFR2 E7090 II
NCT02699606 2 FGRF Erdafitinib II
NCT03212274 2 PARP Olaparib II
NCT03878095 2 PARP Olaparib II
Ceralasertib (AZD6738)
NCT04298021 2 PARP Ceralasertib (AZD6738) + durvalumab or AZD6738 + II
Olaparib
NCT04042831 2 PARP Olaparib II
NCT03521219 2 VEGFR2 Apatinib II
NCT03427242 2 VEGFR2 Apatinib II
NCT02520141 2 VEGF Ramucirumab II
NCT03377179 2 SK2 Opaganib/HCQ II
NCT03185988 2 HER2 Trastuzumab II
NCT04466891 2 HER2 ZW25 (Zanidatamab) II
NCT03833661 2 PD-L1; TGF-β Trap fusion M7824 II
protein
NCT02576431 2 NTRK Larotrectinib II
NCT02568267 2 NTRK Entrectinib II
NCT03207347 2 PARP Niraparib II
NCT02052778 1/2 FGFR Futibatinib (TAS-120) II
NCT03684811 1/2 IDH1 FT-2102 II
NCT04209465 1/2 EGFR/ErbB BDTX-189 II
NCT03422679 1/2 NOTCH CB-103 II
NCT03149549 1/2 TAA CD166 CX-2009 II
NCT02908451 1 TAA AG7 AbGn-107 II
FGF: Fibroblast growth factor; FGFR: fibroblast growth factor receptor; IDH-1: isocitrate dehydrogenase 1; PARP: poly-ADP ribose polymerase; PD-
L1: programmed death ligand 1; TGF-β: transforming growth factor β.
pemigatinib in the second-line setting of CCA with FGFR2 fusions based on the comparison with historical
data. Indeed, mOS for aBTC patients treated with ASC or chemotherapy in second-line is 6 months, as
[8]
highlighted in the ABC-06 and ClarIDHy trial. Even considering the better prognosis of iCCA patients ,
according to the subgroup analysis of the ABC trials, this is expected to be around 16 months from
diagnosis, leaving an expected survival of < 8 months from the starting of second-line treatment providing a
mPFS of 8 months for first-line CisGem [Figure 3]. FGFR2 fusions are also known to be associated with a
better prognosis with a higher number of patients being diagnosed at an early stage which justifies the
difference in OS between patients with and without FGFR2 rearranged CCA in a retrospective multi-
institutional American cohort. In this analysis of 377 patients, mOS from diagnosis was 37 months vs.
20 months in patients with FGFR2-fused tumours (P < 0.01) compared to those without these genetic
abnormalities . Of note, 40% in the FGFR2-fused group underwent surgical resection, likely impacting
[80]
these figures. Indeed, Javle et al. demonstrated that FGFR2-fused CCA undergoing second-line
[81]
chemotherapy are not associated with better response with a PFS of 4.63 months, comparable to the