Page 60 - Read Online
P. 60
Page 4 of 23 Casolino et al. Hepatoma Res 2021;7:76 https://dx.doi.org/10.20517/2394-5079.2021.79
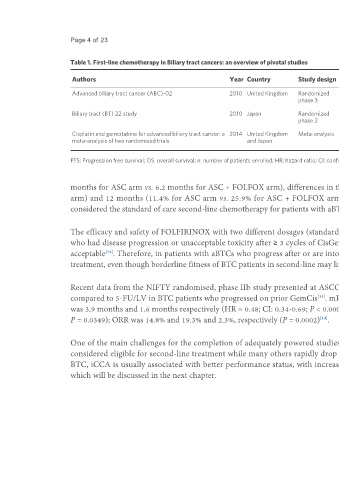
Table 1. First-line chemotherapy in Biliary tract cancers: an overview of pivotal studies
Median PFS Median OS
Authors Year Country Study design Regimen n HR HR
(months) (months)
Advanced biliary tract cancer (ABC)-02 2010 United Kingdom Randomized CisGem 204 8.0 0.63; 95%CI: 0.51- 11.7 0.64; 95%CI: 0.52-
phase 3 0.77, P < 0.001 0.80, P < 0.001
GEM 206 5.0 8.1
Biliary tract (BT) 22 study 2010 Japan Randomized CisGem 41 5.8 0.66; 95%CI: 0.41- 11.2 0.69; 95%CI: 0.42-
phase 2 1.05, P = 0.077 1.13, P = 0.139
GEM 42 3.7 7.7
Cisplatin and gemcitabine for advanced biliary tract cancer: a 2014 United Kingdom Meta-analysis CisGem - 8.8 0.64; 95%CI: 0.53- 11.6 0.65; 95%CI: 0.54-
meta-analysis of two randomised trials and Japan 0.76, P < 0.001 0.78, P < 0.001
GEM - 6.7 8
PFS: Progression free survival; OS: overall survival; n: number of patients enrolled; HR: hazard ratio; CI: confidence interval; CisGem: cisplatin and gemcitabine.
months for ASC arm vs. 6.2 months for ASC + FOLFOX arm), differences in the survival rate at 6 months (35.5% for ASC arm vs. 50.6% for ASC + FOLFOX
arm) and 12 months (11.4% for ASC arm vs. 25.9% for ASC + FOLFOX arm) were clinically meaningful . Based on this evidence, FOLFOX is currently
[8]
considered the standard of care second-line chemotherapy for patients with aBTCs without driver mutations who remains fit following CisGem progression.
The efficacy and safety of FOLFIRINOX with two different dosages (standard and modified) was investigated in a recent phase II trial enrolling 40 patients
who had disease progression or unacceptable toxicity after ≥ 3 cycles of CisGem. The mPFS and mOS were 6.2 and 10.7 months, and the toxicity profile was
acceptable . Therefore, in patients with aBTCs who progress after or are intolerant to CisGem, FOLFIRINOX could be considered as an option for salvage
[34]
treatment, even though borderline fitness of BTC patients in second-line may limit its applications .
[12]
Recent data from the NIFTY randomised, phase IIb study presented at ASCO 2021 showed that Nal-IRI plus 5-FU/LV significantly improved PFS and OS
compared to 5-FU/LV in BTC patients who progressed on prior GemCis . mPFS per investigator review in Nal-IRI plus 5-FU/LV group and 5-FU/LV group
[14]
was 3.9 months and 1.6 months respectively (HR = 0.48; CI: 0.34-0.69; P < 0.0001); mOS was 8.6 months and 5.5 months respectively (HR = 0.68; CI: 0.48-0.98;
[14]
P = 0.0349); ORR was 14.8% and 19.3% and 2.3%, respectively (P = 0.0002) .
One of the main challenges for the completion of adequately powered studies is that, given the aggressive clinical behaviour of CCA, < 40% of patients are
considered eligible for second-line treatment while many others rapidly drop out of clinical trials due to clinical deterioration [35-39] . Amongst the subtypes of
BTC, iCCA is usually associated with better performance status, with increased access to personalised approaches based on the tumour molecular profile,
which will be discussed in the next chapter.