Page 29 - Read Online
P. 29
Page 10 of 15 Feng et al. Hepatoma Res 2021;7:3 I http://dx.doi.org/10.20517/2394-5079.2020.107
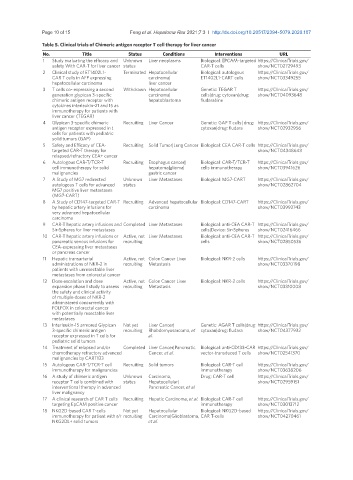
Table 5. Clinical trials of Chimeric antigen receptor T cell therapy for liver cancer
No. Title Status Conditions Interventions URL
1 Study evaluating the efficacy and Unknown Liver neoplasms Biological: EPCAM-targeted https://ClinicalTrials.gov/
safety With CAR-T for liver cancer status CAR-T cells show/NCT02729493
2 Clinical study of ET1402L1- Terminated Hepatocellular Biological: autologous https://ClinicalTrials.gov/
CAR T cells in AFP expressing carcinoma| ET1402L1-CART cells show/NCT03349255
hepatocellular carcinoma liver cancer
3 T cells co- expressing a second Withdrawn Hepatocellular Genetic: TEGAR T https://ClinicalTrials.gov/
generation glypican 3-specific carcinoma| cells|drug: cytoxan|drug: show/NCT04093648
chimeric antigen receptor with hepatoblastoma fludarabine
cytokines interleukin-21 and 15 as
immunotherapy for patients with
liver cancer (TEGAR)
4 Glypican 3-specific chimeric Recruiting Liver Cancer Genetic: GAP T cells| drug: https://ClinicalTrials.gov/
antigen receptor expressed in t cytoxan|drug: fludara show/NCT02932956
cells for patients with pediatric
solid tumors (GAP)
5 Safety and Efficacy of CEA- Recruiting Solid Tumor|Lung Cancer Biological: CEA CAR-T cells https://ClinicalTrials.gov/
targeted CAR-T therapy for show/NCT04348643
relapsed/refractory CEA+ cancer
6 Autologous CAR-T/TCR-T Recruiting Esophagus cancer| Biological: CAR-T/TCR-T https://ClinicalTrials.gov/
cell immunotherapy for solid hepatoma|glioma| cells immunotherapy show/NCT03941626
malignancies gastric cancer
7 A Study of MG7 redirected Unknown Liver Metastases Biological: MG7-CART https://ClinicalTrials.gov/
autologous T cells for advanced status show/NCT02862704
MG7 positive liver metastases
(MG7-CART)
8 A Study of CD147-targeted CAR-T Recruiting Advanced hepatocellular Biological: CD147-CART https://ClinicalTrials.gov/
by hepatic artery infusions for carcinoma show/NCT03993743
very advanced hepatocellular
carcinoma
9 CAR-T hepatic artery infusions and Completed Liver Metastases Biological: anti-CEA CAR-T https://ClinicalTrials.gov/
Sir-Spheres for liver metastases cells|Device: Sir-Spheres show/NCT02416466
10 CAR-T hepatic artery infusions or Active, not Liver Metastases Biological: anti-CEA CAR-T https://ClinicalTrials.gov/
pancreatic venous infusions for recruiting cells show/NCT02850536
CEA-expressing liver metastases
or pancreas cancer
11 Hepatic transarterial Active, not Colon Cancer Liver Biological: NKR-2 cells https://ClinicalTrials.gov/
administrations of NKR-2 in recruiting Metastasis show/NCT03370198
patients with unresectable liver
metastases from colorectal cancer
12 Dose escalation and dose Active, not Colon Cancer Liver Biological: NKR-2 cells https://ClinicalTrials.gov/
expansion phase I study to assess recruiting Metastasis show/NCT03310008
the safety and clinical activity
of multiple doses of NKR-2
administered concurrently with
FOLFOX in colorectal cancer
with potentially resectable liver
metastases
13 Interleukin-15 armored Glypican Not yet Liver Cancer| Genetic: AGAR T cells|drug: https://ClinicalTrials.gov/
3-specific chimeric antigen recruiting Rhabdomyosarcoma, et cytoxan|drug: fludara show/NCT04377932
receptor expressed in T cells for al.
pediatric solid tumors
14 Treatment of relapsed and/or Completed Liver Cancer|Pancreatic Biological: anti-CD133-CAR https://ClinicalTrials.gov/
chemotherapy refractory advanced Cancer, et al. vector-transduced T cells show/NCT02541370
malignancies by CART133
15 Autologous CAR-T/TCR-T cell Recruiting Solid tumors Biological: CAR-T cell https://ClinicalTrials.gov/
immunotherapy for malignancies immunotherapy show/NCT03638206
16 A study of chimeric antigen Unknown Carcinoma, Drug: CAR-T cell https://ClinicalTrials.gov/
receptor T cells combined with status Hepatocellular| show/NCT02959151
interventional therapy in advanced Pancreatic Cancer, et al.
liver malignancy
17 A clinical research of CAR T cells Recruiting Hepatic Carcinoma, et al. Biological: CAR-T cell https://ClinicalTrials.gov/
targeting EpCAM positive cancer immunotherapy show/NCT03013712
18 NKG2D-based CAR T-cells Not yet Hepatocellular Biological: NKG2D-based https://ClinicalTrials.gov/
immunotherapy for patient with r/r recruiting Carcinoma|Glioblastoma, CAR T-cells show/NCT04270461
NKG2DL+ solid tumors et al.