Page 24 - Read Online
P. 24
Feng et al. Hepatoma Res 2021;7:3 I http://dx.doi.org/10.20517/2394-5079.2020.107 Page 5 of 15
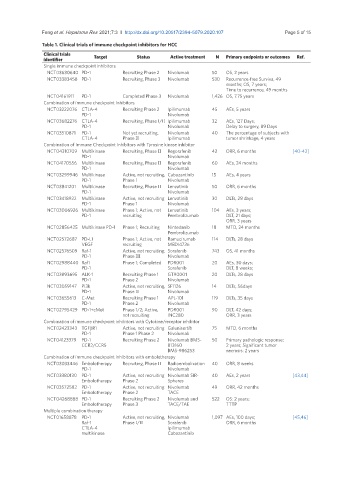
Table 1. Clinical trials of immune checkpoint inhibitors for HCC
Clinical trials
identifier Target Status Active treatment N Primary endpoints or outcomes Ref.
Single immune checkpoint inhibitors
NCT03630640 PD-1 Recruiting Phase 2 Nivolumab 50 OS, 2 years
NCT03383458 PD-1 Recruiting, Phase 3 Nivolumab 530 Recurrence-free Surviva, 49
months; OS, 7 years;
Time to recurrence, 49 months
NCT04161911 PD-1 Completed Phase 3 Nivolumab 1,426 OS, 7.75 years
Combination of immune checkpoint Inhibitors
NCT03222076 CTLA-4 Recruiting Phase 2 Ipilimumab 45 AEs, 5 years
PD-1 Nivolumab
NCT03682276 CTLA-4 Recruiting, Phase I/II Ipilimumab 32 AEs, 127 Days;
PD-1 Nivolumab Delay to surgery, 89 Days
NCT03510871 PD-1 Not yet recruiting, Nivolumab 40 The percentage of subjects with
CTLA-4 Phase II Ipilimumab tumor shrinkage, 4 years
Combination of Immune Checkpoint Inhibitors with Tyrosine kinase inhibitor
NCT04310709 Multikinase Recruiting, Phase II Regorafenib 42 ORR, 6 months [40-42]
PD-1 Nivolumab
NCT04170556 Multikinase Recruiting, Phase II Regorafenib 60 AEs, 24 months
PD-1 Nivolumab
NCT03299946 Multikinase Active, not recruiting, Cabozantinib 15 AEs, 4 years
PD-1 Phase I Nivolumab
NCT03841201 Multikinase Recruiting, Phase II Lenvatinib 50 ORR, 6 months
PD-1 Nivolumab
NCT03418922 Multikinase Active, not recruiting Lenvatinib 30 DLTs, 28 days
PD-1 Phase 1 Nivolumab
NCT03006926 Multikinase Phase 1; Active, not Lenvatinib 104 AEs, 3 years;
PD-1 recruiting Pembrolizumab DLT, 21 days;
ORR, 3 years
NCT02856425 Multikinase PD-1 Phase 1; Recruiting Nintedanib 18 MTD, 24 months
Pembrolizumab
NCT02572687 PD-L1 Phase 1; Active, not Ramucirumab 114 DLTs, 28 days
VEGF recruiting MEDI4736
NCT02576509 Raf-1 Active, not recruiting, Sorafenib 743 OS, 41 months
PD-1 Phase III Nivolumab
NCT02988440 Raf1 Phase 1; Completed PDR001 20 AEs, 30 days;
PD-1 Sorafenib DLT, 8 weeks;
NCT03893695 ALK-1 Recruiting Phase 1 GT90001 20 DLTs, 28 days
PD-1 Phase 2 Nivolumab
NCT03059147 PI3k Active, not recruiting, SF1126 14 DLTs, 56days
PD-1 Phase II Nivolumab
NCT03655613 C-Met Recruiting Phase 1 APL-101 119 DLTs, 35 days
PD-1 Phase 2 Nivolumab
NCT02795429 PD-1+cMet Phase 1/2; Active, PDR001 90 DLT, 42 days;
not recruiting INC280 ORR, 3 years
Combination of immune checkpoint inhibitors with Cytokine/receptor inhibitor
NCT02423343 TGFβR1 Active, not recruiting Galunisertib 75 MTD, 6 months
PD-1 Phase 1 Phase 2 Nivolumab
NCT04123379 PD-1 Recruiting Phase 2 Nivolumab BMS- 50 Primary pathologic response:
CCR2/CCR5 813160 2 years; Significant tumor
BMS-986253 necrosis: 2 years
Combination of immune checkpoint inhibitors with embolotherapy
NCT03033446 Embolotherapy Recruiting, Phase II Radioembolization 40 ORR, 8 weeks
PD-1 Nivolumab
NCT03380130 PD-1 Active, not recruiting Nivolumab SIR- 40 AEs, 2 years [43,44]
Embolotherapy Phase 2 Spheres
NCT03572582 PD-1 Active, not recruiting Nivolumab 49 ORR, 42 months
Embolotherapy Phase 2 TACE
NCT04268888 PD-1 Recruiting Phase 2 Nivolumab and 522 OS: 2 years;
Embolotherapy Phase 3 TACE/TAE TTTP
Multiple combination therapy
NCT01658878 PD-1 Active, not recruiting, Nivolumab 1,097 AEs, 100 days; [45,46]
Raf-1 Phase I/II Sorafenib ORR, 6 months
CTLA-4 Ipilimumab
multikinase Cabozantinib