Page 22 - Read Online
P. 22
Feng et al. Hepatoma Res 2021;7:3 I http://dx.doi.org/10.20517/2394-5079.2020.107 Page 3 of 15
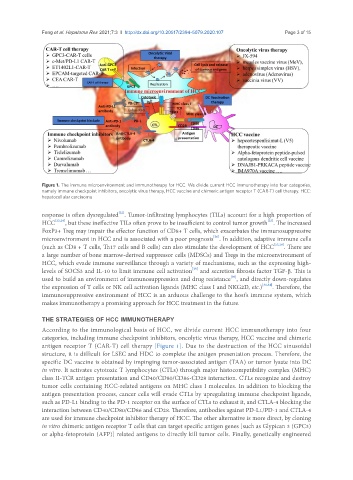
Figure 1. The immune microenvironment and immunotherapy for HCC. We divide current HCC immunotherapy into four categories,
namely immune checkpoint inhibitors, oncolytic virus therapy, HCC vaccine and chimeric antigen receptor T (CAR-T) cell therapy. HCC:
hepatocellular carcinoma
[22]
response is often dysregulated . Tumor-infiltrating lymphocytes (TILs) account for a high proportion of
[25]
HCC [23,24] , but these ineffective TILs often prove to be insufficient to control tumor growth . The increased
FoxP3+ Treg may impair the effector function of CD8+ T cells, which exacerbates the immunosuppressive
[26]
microenvironment in HCC and is associated with a poor prognosis . In addition, adaptive immune cells
(such as CD8 + T cells, Th17 cells and B cells) can also stimulate the development of HCC [27,28] . There are
a large number of bone marrow-derived suppressor cells (MDSCs) and Tregs in the microenvironment of
HCC, which evade immune surveillance through a variety of mechanisms, such as the expressing high-
levels of SOCS3 and IL-10 to limit immune cell activation and secretion fibrosis factor TGF-β. This is
[29]
[30]
used to build an environment of immunosuppression and drug resistance , and directly down-regulates
the expression of T cells or NK cell activation ligands (MHC class I and NKG2D, etc.) [31,32] . Therefore, the
immunosuppressive environment of HCC is an arduous challenge to the host’s immune system, which
makes immunotherapy a promising approach for HCC treatment in the future.
THE STRATEGIES OF HCC IMMUNOTHERAPY
According to the immunological basis of HCC, we divide current HCC immunotherapy into four
categories, including immune checkpoint inhibitors, oncolytic virus therapy, HCC vaccine and chimeric
antigen receptor T (CAR-T) cell therapy [Figure 1]. Due to the destruction of the HCC sinusoidal
structure, it is difficult for LSEC and HDC to complete the antigen presentation process. Therefore, the
specific DC vaccine is obtained by impinging tumor-associated antigen (TAA) or tumor lysate into DC
in vitro. It activates cytotoxic T lymphocytes (CTLs) through major histocompatibility complex (MHC)
class II-TCR antigen presentation and CD40/CD80/CD86-CD28 interaction. CTLs recognize and destroy
tumor cells containing HCC-related antigens on MHC class I molecules. In addition to blocking the
antigen presentation process, cancer cells will evade CTLs by upregulating immune checkpoint ligands,
such as PD-L1 binding to the PD-1 receptor on the surface of CTLs to exhaust it, and CTLA-4 blocking the
interaction between CD40/CD80/CD86 and CD28. Therefore, antibodies against PD-L1/PD-1 and CTLA-4
are used for immune checkpoint inhibitor therapy of HCC. The other alternative is more direct, by cloning
in vitro chimeric antigen receptor T cells that can target specific antigen genes [such as Glypican 3 (GPC3)
or alpha-fetoprotein (AFP)] related antigens to directly kill tumor cells. Finally, genetically engineered