Page 233 - Read Online
P. 233
Shami-shah et al. Extracell Vesicles Circ Nucleic Acids 2023;4:447-60 https://dx.doi.org/10.20517/evcna.2023.14 Page 453
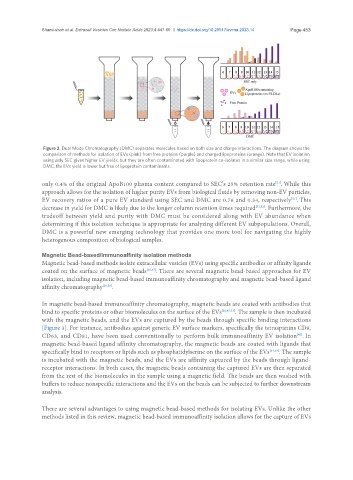
Figure 2. Dual Mode Chromatography (DMC) separates molecules based on both size and charge interactions. The diagram shows the
comparison of methods for isolation of EVs (pink) from free proteins (purple) and charged lipoproteins (orange). Note that EV isolation
using only SEC gives higher EV yields, but they are often contaminated with lipoprotein co-isolates in a similar size range, while using
DMC, the EVs yield is lower but free of lipoprotein contaminants.
only 0.4% of the original ApoB100 plasma content compared to SEC’s 25% retention rate . While this
[51]
approach allows for the isolation of higher purity EVs from biological fluids by removing non-EV particles,
EV recovery ratios of a pure EV standard using SEC and DMC are 0.78 and 0.34, respectively . This
[51]
decrease in yield for DMC is likely due to the longer column retention times required [51,52] . Furthermore, the
tradeoff between yield and purity with DMC must be considered along with EV abundance when
determining if this isolation technique is appropriate for analyzing different EV subpopulations. Overall,
DMC is a powerful new emerging technology that provides one more tool for navigating the highly
heterogenous composition of biological samples.
Magnetic Bead-based/Immunoaffinity isolation methods
Magnetic bead-based methods isolate extracellular vesicles (EVs) using specific antibodies or affinity ligands
coated on the surface of magnetic beads [20,47] . There are several magnetic bead-based approaches for EV
isolation, including magnetic bead-based immunoaffinity chromatography and magnetic bead-based ligand
affinity chromatography [20,53] .
In magnetic bead-based immunoaffinity chromatography, magnetic beads are coated with antibodies that
bind to specific proteins or other biomolecules on the surface of the EVs [20,47,53] . The sample is then incubated
with the magnetic beads, and the EVs are captured by the beads through specific binding interactions
[Figure 3]. For instance, antibodies against generic EV surface markers, specifically the tetraspanins CD9,
CD63, and CD81, have been used conventionally to perform bulk immunoaffinity EV isolation . In
[47]
magnetic bead-based ligand affinity chromatography, the magnetic beads are coated with ligands that
specifically bind to receptors or lipids such as phosphatidylserine on the surface of the EVs [54,55] . The sample
is incubated with the magnetic beads, and the EVs are affinity captured by the beads through ligand-
receptor interactions. In both cases, the magnetic beads containing the captured EVs are then separated
from the rest of the biomolecules in the sample using a magnetic field. The beads are then washed with
buffers to reduce nonspecific interactions and the EVs on the beads can be subjected to further downstream
analysis.
There are several advantages to using magnetic bead-based methods for isolating EVs. Unlike the other
methods listed in this review, magnetic bead-based immunoaffinity isolation allows for the capture of EVs