Page 23 - Read Online
P. 23
Zhu et al. Energy Mater. 2025, 5, 500034 https://dx.doi.org/10.20517/energymater.2024.201 Page 5 of 10
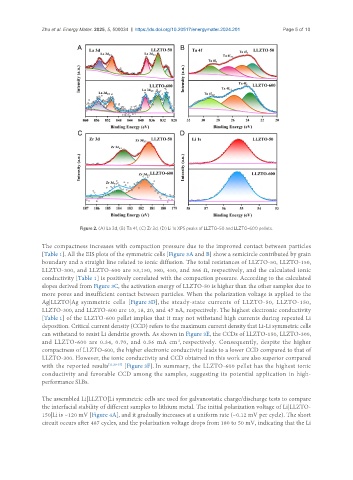
Figure 2. (A) La 3d, (B) Ta 4f, (C) Zr 3d, (D) Li 1s XPS peaks of LLZTO-50 and LLZTO-600 pellets.
The compactness increases with compaction pressure due to the improved contact between particles
[Table 1]. All the EIS plots of the symmetric cells [Figure 3A and B] show a semicircle contributed by grain
boundary and a straight line related to ionic diffusion. The total resistances of LLZTO-50, LLZTO-150,
LLZTO-300, and LLZTO-600 are 53,150, 580, 450, and 366 Ω, respectively, and the calculated ionic
conductivity [Table 1] is positively correlated with the compaction pressure. According to the calculated
slopes derived from Figure 3C, the activation energy of LLZTO-50 is higher than the other samples due to
more pores and insufficient contact between particles. When the polarization voltage is applied to the
Ag|LLZTO|Ag symmetric cells [Figure 3D], the steady-state currents of LLZTO-50, LLZTO-150,
LLZTO-300, and LLZTO-600 are 10, 18, 20, and 47 nA, respectively. The highest electronic conductivity
[Table 1] of the LLZTO-600 pellet implies that it may not withstand high currents during repeated Li
deposition. Critical current density (CCD) refers to the maximum current density that Li-Li symmetric cells
can withstand to resist Li dendrite growth. As shown in Figure 3E, the CCDs of LLZTO-150, LLZTO-300,
-2
and LLZTO-600 are 0.34, 0.70, and 0.56 mA cm , respectively. Consequently, despite the higher
compactness of LLZTO-600, the higher electronic conductivity leads to a lower CCD compared to that of
LLZTO-300. However, the ionic conductivity and CCD obtained in this work are also superior compared
with the reported results [11,30-37] [Figure 3F]. In summary, the LLZTO-600 pellet has the highest ionic
conductivity and favorable CCD among the samples, suggesting its potential application in high-
performance SLBs.
The assembled Li|LLZTO|Li symmetric cells are used for galvanostatic charge/discharge tests to compare
the interfacial stability of different samples to lithium metal. The initial polarization voltage of Li|LLZTO-
150|Li is ~120 mV [Figure 4A], and it gradually increases at a uniform rate (~0.12 mV per cycle). The short
circuit occurs after 487 cycles, and the polarization voltage drops from 180 to 50 mV, indicating that the Li