Page 108 - Read Online
P. 108
Page 8 of 14 Chen et al. Energy Mater. 2025, 5, 500064 https://dx.doi.org/10.20517/energymater.2024.163
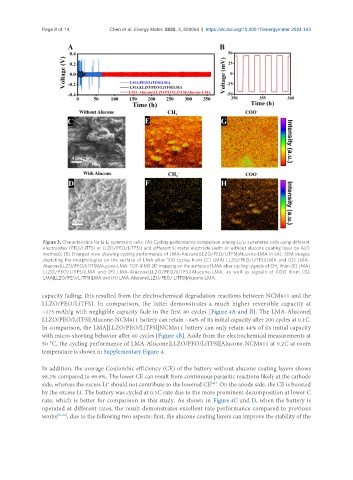
Figure 3. Characteristics for Li-Li symmetric cells: (A) Cycling performance comparison among Li/Li symmetric cells using different
electrolytes (PEO/LiTFSI or LLZO/PEO/LiTFSI) and different Li metal electrode (with or without alucone coating layer by ALD
method); (B) Enlarged view showing cycling performance of LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-LMA in (A). SEM images
depicting the morphologies on the surface of LMA after 100 cycles from (C) LMA| LLZO/PEO/LiTFSI|LMA and (D) LMA-
-
Alucone|LLZO/PEO/LiTFSI|Alucone-LMA. TOF-SIMS 2D mapping on the surface of LMA after cycling: signals of CH from (E) LMA|
x
-
LLZO/PEO/LiTFSI|LMA and (F) LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-LMA, as well as signals of COO from (G)
LMA|LLZO/PEO/LiTFSI|LMA and (H) LMA-Alucone|LLZO/PEO/ LiTFSI|Alucone-LMA.
capacity fading; this resulted from the electrochemical degradation reactions between NCM811 and the
LLZO/PEO/LiTFSI. In comparison, the latter demonstrates a much higher reversible capacity at
~175 mAh/g with negligible capacity fade in the first 80 cycles [Figure 4A and B]. The LMA-Alucone|
LLZO/PEO/LiTFSI|Alucone-NCM811 battery can retain ~84% of its initial capacity after 200 cycles at 0.1C.
In comparison, the LMA|LLZO/PEO/LiTFSI|NCM811 battery can only retain 44% of its initial capacity
with micro-shorting behavior after 80 cycles [Figure 4B]. Aside from the electrochemical measurements at
50 °C, the cycling performance of LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-NCM811 at 0.2C at room
temperature is shown in Supplementary Figure 4.
In addition, the average Coulombic efficiency (CE) of the battery without alucone coating layers shows
98.2% compared to 99.9%. The lower CE can result from continuous parasitic reactions likely at the cathode
+
side, whereas the excess Li should not contribute to the lowered CE . On the anode side, the CE is boosted
[49]
by the excess Li. The battery was cycled at 0.1C rate due to the more prominent decomposition at lower C
rate, which is better for comparison in this study. As shown in Figure 4C and D, when the battery is
operated at different rates, the result demonstrates excellent rate performance compared to previous
works [50-52] , due to the following two aspects: first, the alucone coating layers can improve the stability of the