Page 107 - Read Online
P. 107
Chen et al. Energy Mater. 2025, 5, 500064 https://dx.doi.org/10.20517/energymater.2024.163 Page 7 of 14
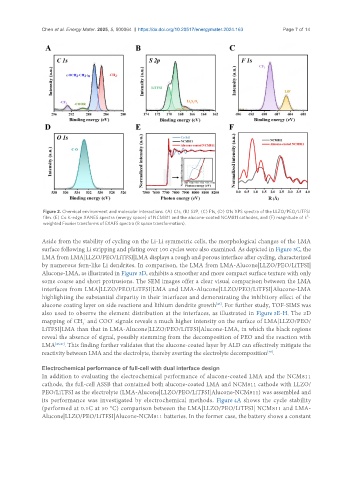
Figure 2. Chemical environment and molecular interactions. (A) C1s, (B) S2P, (C) F1s, (D) O1s XPS spectra of the LLZO/PEO/LiTFSI
3
film. (E) Co K-edge XANES spectra (energy space) of NCM811 and the alucone-coated NCM811 cathodes, and (F) magnitude of k -
weighted Fourier transforms of EXAFS spectra (R space transformation).
Aside from the stability of cycling on the Li-Li symmetric cells, the morphological changes of the LMA
surface following Li stripping and plating over 100 cycles were also examined. As depicted in Figure 3C, the
LMA from LMA|LLZO/PEO/LiTFSI|LMA displays a rough and porous interface after cycling, characterized
by numerous fern-like Li dendrites. In comparison, the LMA from LMA-Alucone|LLZO/PEO/LiTFSI|
Alucone-LMA, as illustrated in Figure 3D, exhibits a smoother and more compact surface texture with only
some coarse and short protrusions. The SEM images offer a clear visual comparison between the LMA
interfaces from LMA|LLZO/PEO/LiTFSI|LMA and LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-LMA
highlighting the substantial disparity in their interfaces and demonstrating the inhibitory effect of the
[45]
alucone coating layer on side reactions and lithium dendrite growth . For further study, TOF-SIMS was
also used to observe the element distribution at the interfaces, as illustrated in Figure 3E-H. The 2D
-
-
mapping of CH and COO signals reveals a much higher intensity on the surface of LMA|LLZO/PEO/
x
LiTFSI|LMA than that in LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-LMA, in which the black regions
reveal the absence of signal, possibly stemming from the decomposition of PEO and the reaction with
LMA [46,47] . This finding further validates that the alucone-coated layer by ALD can effectively mitigate the
[48]
reactivity between LMA and the electrolyte, thereby averting the electrolyte decomposition .
Electrochemical performance of full-cell with dual interface design
In addition to evaluating the electrochemical performance of alucone-coated LMA and the NCM811
cathode, the full-cell ASSB that contained both alucone-coated LMA and NCM811 cathode with LLZO/
PEO/LiTFSI as the electrolyte (LMA-Alucone|LLZO/PEO/LiTFSI|Alucone-NCM811) was assembled and
its performance was investigated by electrochemical methods. Figure 4A shows the cycle stability
(performed at 0.1C at 50 °C) comparison between the LMA|LLZO/PEO/LiTFSI| NCM811 and LMA-
Alucone|LLZO/PEO/LiTFSI|Alucone-NCM811 batteries. In the former case, the battery shows a constant