Page 86 - Read Online
P. 86
Rehman et al. Energy Mater 2024;4:400068 https://dx.doi.org/10.20517/energymater.2024.06 Page 17 of 64
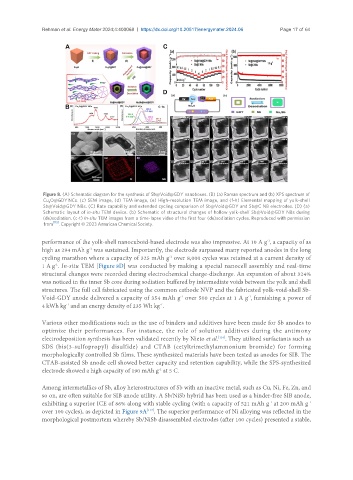
Figure 8. (A) Schematic diagram for the synthesis of Sb@Void@GDY nanoboxes. (B) (a) Raman spectrum and (b) XPS spectrum of
Cu O@GDY NCs. (c) SEM image, (d) TEM image, (e) High-resolution TEM image, and (f-h) Elemental mapping of yolk-shell
2
Sb@Void@GDY NBs. (C) Rate capability and extended cycling comparison of Sb@Void@GDY and Sb@C NB electrodes. (D) (a)
Schematic layout of in-situ TEM device. (b) Schematic of structural changes of hollow yolk-shell Sb@Void@GDY NBs during
(de)sodiation. (c-t) In-situ TEM images from a time-lapse video of the first four (de)sodiation cycles. Reproduced with permission
from [115] . Copyright © 2023 American Chemical Society.
performance of the yolk-shell nanocuboid-based electrode was also impressive. At 10 A g , a capacity of as
-1
high as 294 mAh g was sustained. Importantly, the electrode surpassed many reported anodes in the long
-1
cycling marathon where a capacity of 325 mAh g over 8,000 cycles was retained at a current density of
-1
1 A g . In-situ TEM [Figure 8D] was conducted by making a special nanocell assembly and real-time
-1
structural changes were recorded during electrochemical charge-discharge. An expansion of about 324%
was noticed in the inner Sb core during sodiation buffered by intermediate voids between the yolk and shell
structures. The full cell fabricated using the common cathode NVP and the fabricated yolk-void-shell Sb-
-1
Void-GDY anode delivered a capacity of 354 mAh g over 500 cycles at 1 A g , furnishing a power of
-1
4 kWh kg and an energy density of 235 Wh kg .
-1
-1
Various other modifications such as the use of binders and additives have been made for Sb anodes to
optimize their performances. For instance, the role of solution additives during the antimony
electrodeposition synthesis has been validated recently by Nieto et al. . They utilized surfactants such as
[116]
SDS (bis(3-sulfopropyl) disulfide) and CTAB (cetyltrimethylammonium bromide) for forming
morphologically controlled Sb films. These synthesized materials have been tested as anodes for SIB. The
CTAB-assisted Sb anode cell showed better capacity and retention capability, while the SPS-synthesized
electrode showed a high capacity of 190 mAh g at 5 C.
-1
Among intermetallics of Sb, alloy heterostructures of Sb with an inactive metal, such as Cu, Ni, Fe, Zn, and
so on, are often suitable for SIB anode utility. A Sb/NiSb hybrid has been used as a binder-free SIB anode,
exhibiting a superior ICE of 86% along with stable cycling (with a capacity of 521 mAh g at 200 mAh g
-1
-1
over 100 cycles), as depicted in Figure 9A . The superior performance of Ni alloying was reflected in the
[117]
morphological postmortem whereby Sb/NiSb disassembled electrodes (after 100 cycles) presented a stable,