Page 23 - Read Online
P. 23
Wang et al. Energy Mater 2024;4:400031 https://dx.doi.org/10.20517/energymater.2023.103 Page 7 of 11
-2
-2
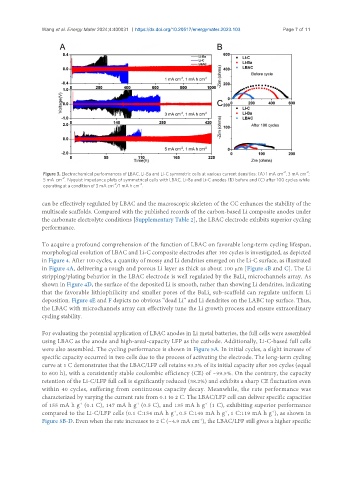
Figure 3. Electrochemical performances of LBAC, Li-Ba and Li-C symmetric cells at various current densities: (A) 1 mA cm ; 3 mA cm ;
-2
5 mA cm . Nyquist impedance plots of symmetrical cells with LBAC, Li-Ba and Li-C anodes (B) before and (C) after 100 cycles while
-2 -2
operating at a condition of 3 mA cm /1 mA h cm .
can be effectively regulated by LBAC and the macroscopic skeleton of the CC enhances the stability of the
multiscale scaffolds. Compared with the published records of the carbon-based Li composite anodes under
the carbonate electrolyte conditions [Supplementary Table 2], the LBAC electrode exhibits superior cycling
performance.
To acquire a profound comprehension of the function of LBAC on favorable long-term cycling lifespan,
morphological evolution of LBAC and Li-C composite electrodes after 100 cycles is investigated, as depicted
in Figure 4. After 100 cycles, a quantity of mossy and Li dendrites emerged on the Li-C surface, as illustrated
in Figure 4A, delivering a rough and porous Li layer as thick as about 100 μm [Figure 4B and C]. The Li
stripping/plating behavior in the LBAC electrode is well regulated by the BaLi microchannels array. As
4
shown in Figure 4D, the surface of the deposited Li is smooth, rather than showing Li dendrites, indicating
that the favorable lithiophilicity and smaller pores of the BaLi sub-scaffold can regulate uniform Li
4
deposition. Figure 4E and F depicts no obvious “dead Li” and Li dendrites on the LABC top surface. Thus,
the LBAC with microchannels array can effectively tune the Li growth process and ensure extraordinary
cycling stability.
For evaluating the potential application of LBAC anodes in Li metal batteries, the full cells were assembled
using LBAC as the anode and high-areal-capacity LFP as the cathode. Additionally, Li-C-based full cells
were also assembled. The cycling performance is shown in Figure 5A. In initial cycles, a slight increase of
specific capacity occurred in two cells due to the process of activating the electrode. The long-term cycling
curve at 1 C demonstrates that the LBAC/LFP cell retains 93.3% of its initial capacity after 300 cycles (equal
to 600 h), with a consistently stable coulombic efficiency (CE) of ~99.5%. On the contrary, the capacity
retention of the Li-C/LFP full cell is significantly reduced (38.2%) and exhibits a sharp CE fluctuation even
within 40 cycles, suffering from continuous capacity decay. Meanwhile, the rate performance was
characterized by varying the current rate from 0.1 to 2 C. The LBAC/LFP cell can deliver specific capacities
of 155 mA h g (0.1 C), 147 mA h g (0.5 C), and 135 mA h g (1 C), exhibiting superior performance
-1
-1
-1
compared to the Li-C/LFP cells (0.1 C:154 mA h g , 0.5 C:140 mA h g , 1 C:119 mA h g ), as shown in
-1
-1
-1
Figure 5B-D. Even when the rate increases to 2 C (~4.9 mA cm ), the LBAC/LFP still gives a higher specific
-2