Page 91 - Read Online
P. 91
Page 24 of 32 Yan et al. Energy Mater 2023;3:300002 https://dx.doi.org/10.20517/energymater.2022.60
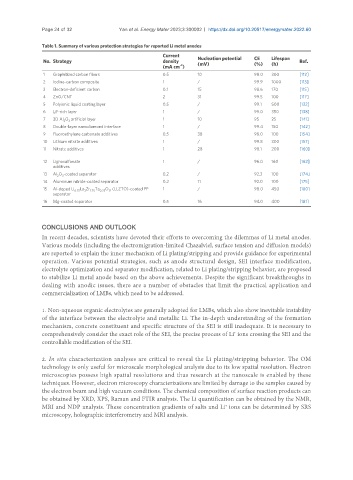
Table 1. Summary of various protection strategies for reported Li metal anodes
Current
No. Strategy density Nucleation potential CE Lifespan Ref.
(mV)
(h)
(%)
-2
(mA cm )
1 Graphitized carbon fibers 0.5 10 98.0 300 [112]
2 Iodine-carbon composite 1 / 99.9 1000 [113]
3 Electron-deficient carbon 0.1 15 98.6 170 [115]
4 ZnO/CNT 2 31 99.5 100 [117]
5 Polyionic liquid coating layer 0.5 / 99.1 500 [132]
6 LiF-rich layer 1 / 99.0 350 [138]
7 3D Al O artificial layer 1 10 95 25 [141]
3
2
8 Double-layer nanodiamond interface 1 / 99.4 150 [142]
9 Fluoroethylene carbonate additives 0.5 38 98.0 100 [154]
10 Lithium nitrate additives 1 / 99.8 300 [157]
11 Nitrate additives 1 28 98.1 200 [160]
12 Lignosulfonate 1 / 96.0 160 [162]
additives
13 Al O -coated separator 0.2 / 92.3 100 [174]
2 3
14 Aluminum nitride-coated separator 0.2 11 92.0 100 [175]
15 Al-doped Li La Zr Ta O (LLZTO)-coated PP 1 / 98.0 450 [180]
6.75 3 1.75 0.25 12
separator
16 Mg-coated separator 0.5 16 94.0 400 [181]
CONCLUSIONS AND OUTLOOK
In recent decades, scientists have devoted their efforts to overcoming the dilemmas of Li metal anodes.
Various models (including the electromigration-limited Chazalviel, surface tension and diffusion models)
are reported to explain the inner mechanism of Li plating/stripping and provide guidance for experimental
operation. Various potential strategies, such as anode structural design, SEI interface modification,
electrolyte optimization and separator modification, related to Li plating/stripping behavior, are proposed
to stabilize Li metal anode based on the above achievements. Despite the significant breakthroughs in
dealing with anodic issues, there are a number of obstacles that limit the practical application and
commercialization of LMBs, which need to be addressed.
1. Non-aqueous organic electrolytes are generally adopted for LMBs, which also show inevitable instability
of the interface between the electrolyte and metallic Li. The in-depth understanding of the formation
mechanism, concrete constituent and specific structure of the SEI is still inadequate. It is necessary to
comprehensively consider the exact role of the SEI, the precise process of Li ions crossing the SEI and the
+
controllable modification of the SEI.
2. In situ characterization analyses are critical to reveal the Li plating/stripping behavior. The OM
technology is only useful for microscale morphological analysis due to its low spatial resolution. Electron
microscopies possess high spatial resolutions and thus research at the nanoscale is enabled by these
techniques. However, electron microscopy characterizations are limited by damage to the samples caused by
the electron beam and high vacuum conditions. The chemical composition of surface reaction products can
be obtained by XRD, XPS, Raman and FTIR analysis. The Li quantification can be obtained by the NMR,
MRI and NDP analysis. These concentration gradients of salts and Li ions can be determined by SRS
+
microscopy, holographic interferometry and MRI analysis.