Page 70 - Read Online
P. 70
Yan et al. Energy Mater 2023;3:300002 https://dx.doi.org/10.20517/energymater.2022.60 Page 3 of 32
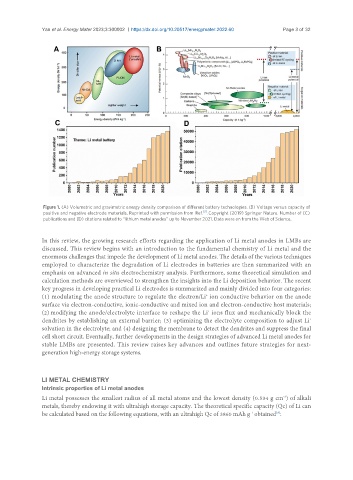
Figure 1. (A) Volumetric and gravimetric energy density comparison of different battery technologies. (B) Voltage versus capacity of
[3]
positive and negative electrode materials. Reprinted with permission from Ref. . Copyright (2019) Springer Nature. Number of (C)
publications and (D) citations related to ‘‘lithium metal anodes’’ up to November 2021. Data were on from the Web of Science.
In this review, the growing research efforts regarding the application of Li metal anodes in LMBs are
discussed. This review begins with an introduction to the fundamental chemistry of Li metal and the
enormous challenges that impede the development of Li metal anodes. The details of the various techniques
employed to characterize the degradation of Li electrodes in batteries are then summarized with an
emphasis on advanced in situ electrochemistry analysis. Furthermore, some theoretical simulation and
calculation methods are overviewed to strengthen the insights into the Li deposition behavior. The recent
key progress in developing practical Li electrodes is summarized and mainly divided into four categories:
+
(1) modulating the anode structure to regulate the electron/Li ion conductive behavior on the anode
surface via electron-conductive, ionic-conductive and mixed ion and electron-conductive host materials;
(2) modifying the anode/electrolyte interface to reshape the Li ions flux and mechanically block the
+
dendrites by establishing an external barrier; (3) optimizing the electrolyte composition to adjust Li
+
solvation in the electrolyte; and (4) designing the membrane to detect the dendrites and suppress the final
cell short circuit. Eventually, further developments in the design strategies of advanced Li metal anodes for
stable LMBs are presented. This review raises key advances and outlines future strategies for next-
generation high-energy storage systems.
LI METAL CHEMISTRY
Intrinsic properties of Li metal anodes
Li metal possesses the smallest radius of all metal atoms and the lowest density (0.534 g cm ) of alkali
-3
metals, thereby endowing it with ultrahigh storage capacity. The theoretical specific capacity (Qc) of Li can
be calculated based on the following equations, with an ultrahigh Qc of 3860 mAh g obtained :
-1
[8]