Page 73 - Read Online
P. 73
Page 6 of 32 Yan et al. Energy Mater 2023;3:300002 https://dx.doi.org/10.20517/energymater.2022.60
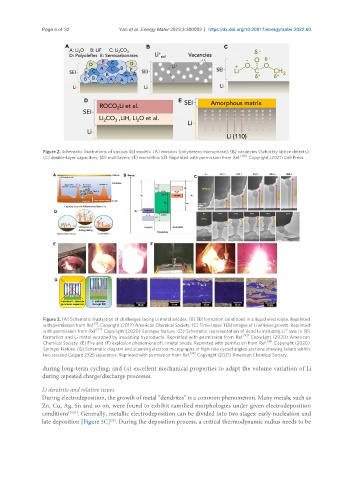
Figure 2. Schematic illustrations of various SEI models: (A) mosaics (polyhetero microphase); (B) vacancies (Schottky lattice defects);
(C) double-layer capacitors; (D) multilayers; (E) monolithic SEI. Reprinted with permission from Ref. [126] . Copyright (2021) Cell Press.
Figure 3. (A) Schematic illustration of challenges facing Li metal anodes. (B) SEI formation conditions in a liquid electrolyte. Reprinted
[8]
with permission from Ref. . Copyright (2017) American Chemical Society. (C) Time-lapse TEM images of Li whisker growth. Reprinted
+
with permission from Ref. [22] . Copyright (2020) Springer Nature. (D) Schematic representation of dead Li including Li ions in SEI
formation and Li metal wrapped by insulating byproducts. Reprinted with permission from Ref. [92] . Copyright (2020) American
Chemical Society. (E) Fire and (F) explosion phenomena of Li metal anode. Reprinted with permission from Ref. [38] . Copyright (2020)
Springer Nature. (G) Schematic diagram and scanning electron micrographs of high-rate cycled angled sections showing failure within
two stacked Celgard 2325 separators. Reprinted with permission from Ref. [39] . Copyright (2021) American Chemical Society.
during long-term cycling; and (4) excellent mechanical properties to adapt the volume variation of Li
during repeated charge/discharge processes.
Li dendrite and relative issues
During electrodeposition, the growth of metal “dendrites” is a common phenomenon. Many metals, such as
Zn, Cu, Ag, Sn and so on, were found to exhibit ramified morphologies under given electrodeposition
conditions [20,21] . Generally, metallic electrodeposition can be divided into two stages: early nucleation and
late deposition [Figure 3C] . During the deposition process, a critical thermodynamic radius needs to be
[22]