Page 31 - Read Online
P. 31
Tao et al. Energy Mater 2022;2:200036 https://dx.doi.org/10.20517/energymater.2022.46 Page 15 of 35
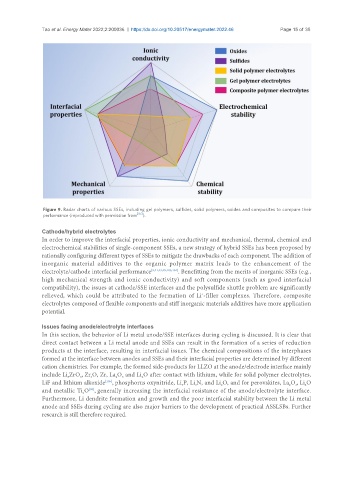
Figure 9. Radar charts of various SSEs, including gel polymers, sulfides, solid polymers, oxides and composites to compare their
performance (reproduced with permission from [97] ).
Cathode/hybrid electrolytes
In order to improve the interfacial properties, ionic conductivity and mechanical, thermal, chemical and
electrochemical stabilities of single-component SSEs, a new strategy of hybrid SSEs has been proposed by
rationally configuring different types of SSEs to mitigate the drawbacks of each component. The addition of
inorganic material additives to the organic polymer matrix leads to the enhancement of the
electrolyte/cathode interfacial performance [9,21,23,45,103,104] . Benefitting from the merits of inorganic SSEs (e.g.,
high mechanical strength and ionic conductivity) and soft components (such as good interfacial
compatibility), the issues at cathode/SSE interfaces and the polysulfide shuttle problem are significantly
+
relieved, which could be attributed to the formation of Li -filler complexes. Therefore, composite
electrolytes composed of flexible components and stiff inorganic materials additives have more application
potential.
Issues facing anode/electrolyte interfaces
In this section, the behavior of Li metal anode/SSE interfaces during cycling is discussed. It is clear that
direct contact between a Li metal anode and SSEs can result in the formation of a series of reduction
products at the interface, resulting in interfacial issues. The chemical compositions of the interphases
formed at the interface between anodes and SSEs and their interfacial properties are determined by different
cation chemistries. For example, the formed side-products for LLZO at the anode/electrode interface mainly
include Li ZrO , Zr O, Zr, La O and Li O after contact with lithium, while for solid polymer electrolytes,
6
3
2
3
2
8
LiF and lithium alkoxide , phosphorus oxynitride, Li P, Li N, and Li O, and for perovskites, La O , Li O
[105]
3
2
3
2
3
2
and metallic Ti O , generally increasing the interfacial resistance of the anode/electrolyte interface.
[90]
6
Furthermore, Li dendrite formation and growth and the poor interfacial stability between the Li metal
anode and SSEs during cycling are also major barriers to the development of practical ASSLSBs. Further
research is still therefore required.