Page 26 - Read Online
P. 26
Page 10 of 35 Tao et al. Energy Mater 2022;2:200036 https://dx.doi.org/10.20517/energymater.2022.46
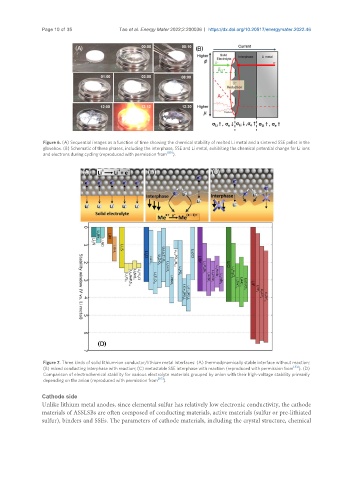
Figure 6. (A) Sequential images as a function of time showing the chemical stability of melted Li metal and a sintered SSE pellet in the
glovebox. (B) Schematic of three phases, including the interphase, SSE and Li metal, exhibiting the chemical potential change for Li ions
[80]
and electrons during cycling (reproduced with permission from ).
Figure 7. Three kinds of solid lithium-ion conductor/lithium metal interfaces: (A) thermodynamically stable interface without reaction;
(B) mixed conducting interphase with reaction; (C) metastable SSE interphase with reaction (reproduced with permission from [81] ). (D)
Comparison of electrochemical stability for various electrolyte materials grouped by anion with their high-voltage stability primarily
depending on the anion (reproduced with permission from [82] ).
Cathode side
Unlike lithium metal anodes, since elemental sulfur has relatively low electronic conductivity, the cathode
materials of ASSLSBs are often composed of conducting materials, active materials (sulfur or pre-lithiated
sulfur), binders and SSEs. The parameters of cathode materials, including the crystal structure, chemical