Page 165 - Read Online
P. 165
Liu et al. Energy Mater 2023;3:300011 https://dx.doi.org/10.20517/energymater.2022.68 Page 7 of 10
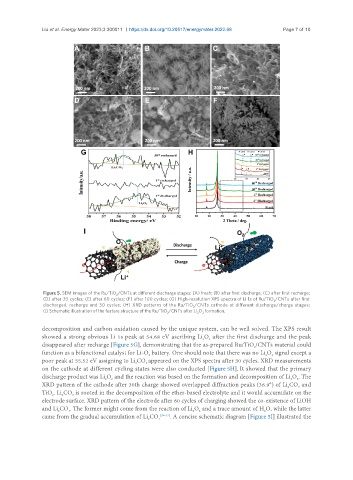
Figure 5. SEM images of the Ru/TiO /CNTs at different discharge stages: (A) fresh; (B) after first discharge; (C) after first recharge;
2
(D) after 30 cycles; (E) after 60 cycles; (F) after 100 cycles; (G) High-resolution XPS spectra of Li 1s of Ru/TiO /CNTs after first
2
discharged, recharge and 30 cycles; (H) XRD patterns of the Ru/TiO /CNTs cathode at different discharge/charge stages;
2
(I) Schematic illustration of the feature structure of the Ru/TiO /CNTs after Li O formation.
2
2
2
decomposition and carbon oxidation caused by the unique system, can be well solved. The XPS result
showed a strong obvious Li 1s peak at 54.68 eV ascribing Li O after the first discharge and the peak
2
2
disappeared after recharge [Figure 5G], demonstrating that the as-prepared Ru/TiO /CNTs material could
2
function as a bifunctional catalyst for Li-O battery. One should note that there was no Li O signal except a
2
2
2
poor peak at 55.52 eV assigning to Li CO appeared on the XPS spectra after 30 cycles. XRD measurements
2
3
on the cathode at different cycling states were also conducted [Figure 5H]. It showed that the primary
discharge product was Li O and the reaction was based on the formation and decomposition of Li O . The
2
2
2
2
XRD pattern of the cathode after 30th charge showed overlapped diffraction peaks (36.9°) of Li CO and
2
3
TiO . Li CO is rooted in the decomposition of the ether-based electrolyte and it would accumulate on the
2
2
3
electrode surface. XRD pattern of the electrode after 60 cycles of charging showed the co-existence of LiOH
and Li CO . The former might come from the reaction of Li O and a trace amount of H O, while the latter
3
2
2
2
2
came from the gradual accumulation of Li CO 3 [35-37] . A concise schematic diagram [Figure 5I] illustrated the
2