Page 156 - Read Online
P. 156
Page 4 of 6 Glushenkov. Energy Mater 2023;3:300010 https://dx.doi.org/10.20517/energymater.2022.70
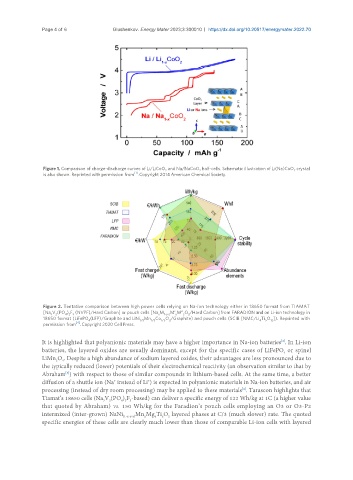
Figure 1. Comparison of charge-discharge curves of Li/LiCoO and Na/NaCoO half-cells. Schematic illustration of Li(Na)CoO crystal
2 2 2
[1]
is also shown. Reprinted with permission from . Copyright 2014 American Chemical Society.
Figure 2. Tentative comparison between high power cells relying on Na-ion technology either in 18650 format from TIAMAT
[Na V (PO ) F (NVPF)/Hard Carbon] or pouch cells [Na M M′ M″ O /Hard Carbon] from FARADION and on Li-ion technology in
3 2 4 2 3 x 1-y-z y z 2
18650 format (LiFePO (LFP)/Graphite and LiNi Mn Co O /Graphite) and pouch cells (SCIB [NMC/Li Ti O ]). Reprinted with
4 1/3 1/3 1/3 2 4 5 12
[4]
permission from . Copyright 2020 Cell Press.
It is highlighted that polyanionic materials may have a higher importance in Na-ion batteries . In Li-ion
[4]
batteries, the layered oxides are usually dominant, except for the specific cases of LiFePO or spinel
4
LiMn O . Despite a high abundance of sodium layered oxides, their advantages are less pronounced due to
4
2
the typically reduced (lower) potentials of their electrochemical reactivity (an observation similar to that by
Abraham ) with respect to those of similar compounds in lithium-based cells. At the same time, a better
[3]
diffusion of a shuttle ion (Na instead of Li ) is expected in polyanionic materials in Na-ion batteries, and air
+
+
[4]
processing (instead of dry room processing) may be applied to these materials . Tarascon highlights that
Tiamat’s 18650 cells (Na V (PO ) F -based) can deliver a specific energy of 122 Wh/kg at 1C (a higher value
2
3
4 2 3
that quoted by Abraham) vs. 150 Wh/kg for the Faradion’s pouch cells employing an O3 or O3-P2
intermixed (inter-grown) NaNi (1-x-y-z) Mn Mg Ti O layered phases at C/3 (much slower) rate. The quoted
2
x
y
z
specific energies of these cells are clearly much lower than those of comparable Li-ion cells with layered