Page 107 - Read Online
P. 107
Page 8 of 13 Park et al. Energy Mater 2023;3:300005 https://dx.doi.org/10.20517/energymater.2022.65
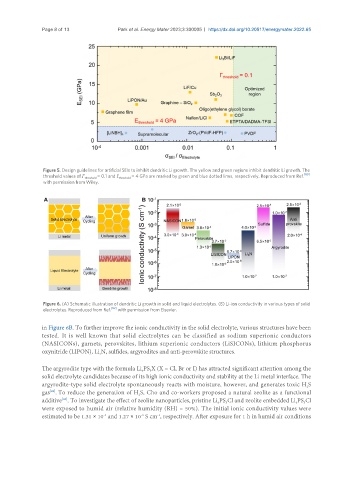
Figure 5. Design guidelines for artificial SEIs to inhibit dendritic Li growth. The yellow and green regions inhibit dendritic Li growth. The
threshold values of Γ threshold = 0.1 and E threshold = 4 GPa are marked by green and blue dotted lines, respectively. Reproduced from Ref. [80]
with permission from Wiley.
Figure 6. (A) Schematic illustration of dendritic Li growth in solid and liquid electrolytes. (B) Li-ion conductivity in various types of solid
electrolytes. Reproduced from Ref. [82] with permission from Elsevier.
in Figure 6B. To further improve the ionic conductivity in the solid electrolyte, various structures have been
tested. It is well known that solid electrolytes can be classified as sodium superionic conductors
(NASICONs), garnets, perovskites, lithium superionic conductors (LiSICONs), lithium phosphorus
oxynitride (LIPON), Li N, sulfides, argyrodites and anti-perovskite structures.
3
The argyrodite type with the formula Li PS X (X = Cl, Br or I) has attracted significant attention among the
5
6
solid electrolyte candidates because of its high ionic conductivity and stability at the Li metal interface. The
argyrodite-type solid electrolyte spontaneously reacts with moisture, however, and generates toxic H S
2
[82]
gas . To reduce the generation of H S, Cho and co-workers proposed a natural zeolite as a functional
2
additive . To investigate the effect of zeolite nanoparticles, pristine Li PS Cl and zeolite embedded Li PS Cl
[83]
5
6
5
6
were exposed to humid air (relative humidity (RH) = 50%). The initial ionic conductivity values were
estimated to be 1.31 × 10 and 1.27 × 10 S cm , respectively. After exposure for 1 h in humid air conditions
-3
-3
-1