Page 105 - Read Online
P. 105
Page 6 of 13 Park et al. Energy Mater 2023;3:300005 https://dx.doi.org/10.20517/energymater.2022.65
Table 1. Ionic conductivity of natural SEI and artificial SEI components
SEI components Ionic conductivity (S cm ) Ref.
-1
-6
LiF 6.0 × 10 -5.2 × 10 -10 [50,51]
-6 -7
LiF-Li CO 3.0 × 10 -3.0 × 10 [52]
2 3
-9 -12
Li O 10 -10 [50,53]
2
Li CO 3 6.7 × 10 -8 [52]
2
-9
Li EDC 4.5 × 10 [54]
2
Li N 1.2 × 10 -4 [55]
3
PEO -5%Li Si 5 3.9 × 10 -5 [56]
m
21
-6
LiZrO(NO ) 2.3 × 10 [57]
3 2
LiAl O 8 3.2 × 10 -6 [58]
5
-6
SPVA 1.59 × 10 [59]
-4
Li P 10 [60]
3
LiRAP film 10 -4 [44]
-5
LiF/Li Sb-5 layer 1.01 × 10 [61]
3
[LiNBH] n 6.6 × 10 -6 [62]
-5
Li S 10 [63]
2
Li AlF 6 10 -5 [64]
3
-5
FE-Li/Na 1.1 × 10 [65]
-6
FE-Li 4.57 × 10 [65]
-6
LIPON 1.1 × 10 -1.4 × 10 -6 [46,66]
Li EDC: Dilithium ethylene decarbonate; PEO: poly(ethylene oxide); SPVA: sulfonated poly(vinyl alcohol); LiRAP: lithium-rich anti-perovskite;
2
FE: fluorinated etching; LIPON: lithium phosphorus oxynitride.
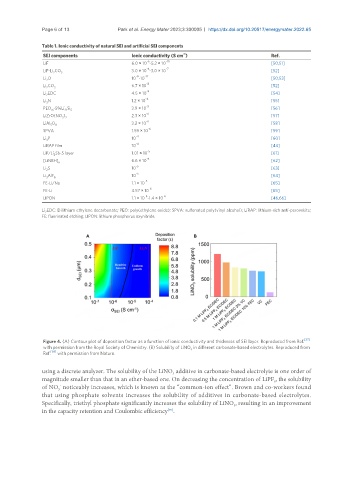
Figure 4. (A) Contour plot of deposition factor as a function of ionic conductivity and thickness of SEI layer. Reproduced from Ref. [37]
with permission from the Royal Society of Chemistry. (B) Solubility of LiNO in different carbonate-based electrolytes. Reproduced from
3
Ref. [69] with permission from Nature.
using a discrete analyzer. The solubility of the LiNO additive in carbonate-based electrolyte is one order of
3
magnitude smaller than that in an ether-based one. On decreasing the concentration of LiPF , the solubility
6
-
of NO noticeably increases, which is known as the “common-ion effect”. Brown and co-workers found
3
that using phosphate solvents increases the solubility of additives in carbonate-based electrolytes.
Specifically, triethyl phosphate significantly increases the solubility of LiNO , resulting in an improvement
3
in the capacity retention and Coulombic efficiency .
[70]