Page 81 - Read Online
P. 81
Page 8 of 33 Mao et al. Chem Synth 2023;3:26 https://dx.doi.org/10.20517/cs.2022.41
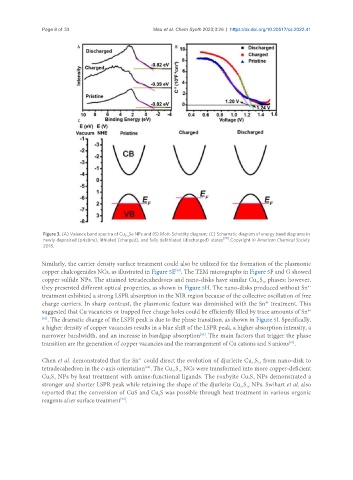
Figure 3. (A) Valence band spectra of Cu Se NPs and (B) Mott-Schottky diagram; (C) Schematic diagram of energy band diagrams in
2-x
newly deposited (pristine), lithiated (charged), and fully delithiated (discharged) states [70] . Copyright © American Chemical Society
2018.
Similarly, the carrier density surface treatment could also be utilized for the formation of the plasmonic
copper chalcogenides NCs, as illustrated in Figure 5E . The TEM micrographs in Figure 5F and G showed
[68]
copper sulfide NPs. The attained tetradecahedrons and nano-disks have similar Cu S phases; however,
31 16
they presented different optical properties, as shown in Figure 5H. The nano-disks produced without Sn
4+
treatment exhibited a strong LSPR absorption in the NIR region because of the collective oscillation of free
charge carriers. In sharp contrast, the plasmonic feature was diminished with the Sn treatment. This
4+
suggested that Cu vacancies or trapped free charge holes could be efficiently filled by trace amounts of Sn 4+
[81] . The dramatic change of the LSPR peak is due to the phase transition, as shown in Figure 5I. Specifically,
a higher density of copper vacancies results in a blue shift of the LSPR peak, a higher absorption intensity, a
narrower bandwidth, and an increase in bandgap absorption . The main factors that trigger the phase
[82]
transition are the generation of copper vacancies and the rearrangement of Cu cations and S anions .
[83]
Chen et al. demonstrated that the Sn could direct the evolution of djurleite Cu S from nano-disk to
4+
31 16
tetradecahedron in the c-axis orientation . The Cu S NCs were transformed into more copper-deficient
[68]
31 16
Cu S NPs by heat treatment with amine-functional ligands. The roxbyite Cu S NPs demonstrated a
7 4
7 4
stronger and shorter LSPR peak while retaining the shape of the djurleite Cu S NPs. Swihart et al. also
31 16
reported that the conversion of CuS and Cu S was possible through heat treatment in various organic
2
reagents after surface treatment .
[79]