Page 51 - Read Online
P. 51
Sun et al. Chem Synth 2023;3:16 https://dx.doi.org/10.20517/cs.2022.45 Page 9 of 17
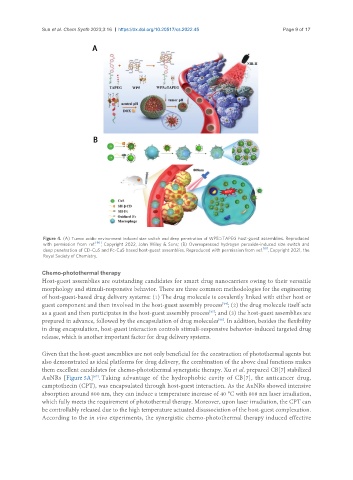
Figure 4. (A) Tumor acidic environment induced size switch and deep penetration of WP5⊃TAPEG host-guest assemblies. Reproduced
with permission from ref. [60] , Copyright 2022, John Wiley & Sons; (B) Overexpressed hydrogen peroxide-induced size switch and
deep penetration of CD-CuS and Fc-CuS based host-guest assemblies. Reproduced with permission from ref. [61] , Copyright 2021, the
Royal Society of Chemistry.
Chemo-photothermal therapy
Host-guest assemblies are outstanding candidates for smart drug nanocarriers owing to their versatile
morphology and stimuli-responsive behavior. There are three common methodologies for the engineering
of host-guest-based drug delivery systems: (1) The drug molecule is covalently linked with either host or
guest component and then involved in the host-guest assembly process ; (2) the drug molecule itself acts
[64]
as a guest and then participates in the host-guest assembly process ; and (3) the host-guest assemblies are
[65]
[66]
prepared in advance, followed by the encapsulation of drug molecules . In addition, besides the flexibility
in drug encapsulation, host-guest interaction controls stimuli-responsive behavior-induced targeted drug
release, which is another important factor for drug delivery systems.
Given that the host-guest assemblies are not only beneficial for the construction of photothermal agents but
also demonstrated as ideal platforms for drug delivery, the combination of the above dual functions makes
them excellent candidates for chemo-photothermal synergistic therapy. Xu et al. prepared CB[7] stabilized
AuNRs [Figure 5A] . Taking advantage of the hydrophobic cavity of CB[7], the anticancer drug,
[67]
camptothecin (CPT), was encapsulated through host-guest interaction. As the AuNRs showed intensive
absorption around 800 nm, they can induce a temperature increase of 40 °C with 808 nm laser irradiation,
which fully meets the requirement of photothermal therapy. Moreover, upon laser irradiation, the CPT can
be controllably released due to the high temperature actuated disassociation of the host-guest complexation.
According to the in vivo experiments, the synergistic chemo-photothermal therapy induced effective