Page 60 - Read Online
P. 60
Page 24 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
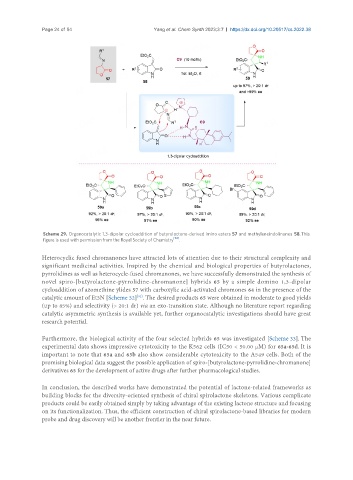
Scheme 29. Organocatalytic 1,3-dipolar cycloaddition of butyrolactone-derived imino esters 57 and methyleneindolinones 58. This
[88]
figure is used with permission from the Royal Society of Chemistry .
Heterocyclic fused chromanones have attracted lots of attention due to their structural complexity and
significant medicinal activities. Inspired by the chemical and biological properties of butyrolactones,
pyrrolidines as well as heterocycle-fused chromanones, we have successfully demonstrated the synthesis of
novel spiro-[butyrolactone-pyrrolidine-chromanone] hybrids 65 by a simple domino 1,3-dipolar
cycloaddition of azomethine ylides 57 with carboxylic acid-activated chromones 64 in the presence of the
catalytic amount of Et3N [Scheme 32] . The desired products 65 were obtained in moderate to good yields
[91]
(up to 85%) and selectivity (> 20:1 dr) via an exo-transition state. Although no literature report regarding
catalytic asymmetric synthesis is available yet, further organocatalytic investigations should have great
research potential.
Furthermore, the biological activity of the four selected hybrids 65 was investigated [Scheme 33]. The
experimental data shows impressive cytotoxicity to the K562 cells (IC50 < 50.00 μM) for 65a-65d. It is
important to note that 65a and 65b also show considerable cytotoxicity to the A549 cells. Both of the
promising biological data suggest the possible application of spiro-[butyrolactone-pyrrolidine-chromanone]
derivatives 65 for the development of active drugs after further pharmacological studies.
In conclusion, the described works have demonstrated the potential of lactone-related frameworks as
building blocks for the diversity-oriented synthesis of chiral spirolactone skeletons. Various complicate
products could be easily obtained simply by taking advantage of the existing lactone structure and focusing
on its functionalization. Thus, the efficient construction of chiral spirolactone-based libraries for modern
probe and drug discovery will be another frontier in the near future.