Page 56 - Read Online
P. 56
Page 20 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
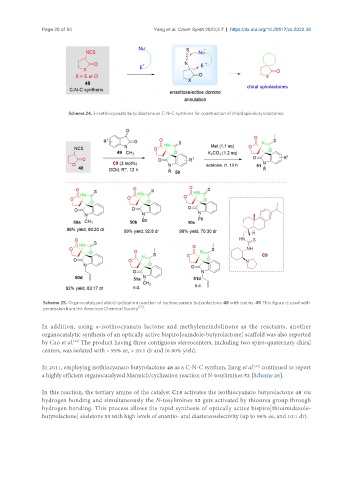
Scheme 24. 3-Isothiocyanato butyrolactone as C-N-C synthons for construction of chiral spirobutyrolactones.
Scheme 25. Organocatalyzed aldol/cyclization reaction of isothiocyanato butyrolactone 48 with isatins 49. This figure is used with
permission from the American Chemical Society [72] .
In addition, using α-isothiocyanato lactone and methyleneindolinone as the reactants, another
organocatalytic synthesis of an optically active bispiro[oxindole-butyrolactone] scaffold was also reported
by Cao et al. The product having three contiguous stereocenters, including two spiro-quaternary chiral
[53]
centers, was isolated with > 99% ee, > 20:1 dr and in 80% yield.
In 2011, employing isothiocyanato butyrolactone 48 as a C-N-C synthon, Jiang et al. continued to report
[80]
a highly efficient organocatalyzed Mannich/cyclization reaction of N-tosylimines 52 [Scheme 26].
In this reaction, the tertiary amine of the catalyst C18 activates the isothiocyanato butyrolactone 48 via
hydrogen bonding and simultaneously the N-tosylimines 52 gets activated by thiourea group through
hydrogen bonding. This process allows the rapid synthesis of optically active bispiro[thioimidazole-
butyrolactone] skeletons 53 with high levels of enantio- and diastereoselectivity (up to 96% ee, and 10:1 dr).