Page 54 - Read Online
P. 54
Page 18 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
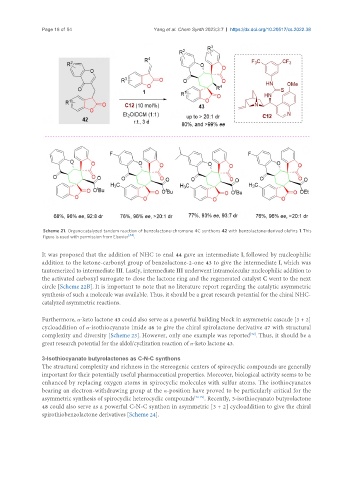
Scheme 21. Organocatalyzed tandem reaction of benzolactone-chromone 4C synthons 42 with benzolactone-derived olefins 1. This
figure is used with permission from Elsevier [64] .
It was proposed that the addition of NHC to enal 44 gave an intermediate I, followed by nucleophilic
addition to the ketone-carbonyl group of benzolactone-2-one 43 to give the intermediate I, which was
tautomerized to intermediate III. Lastly, intermediate III underwent intramolecular nucleophilic addition to
the activated carboxyl surrogate to close the lactone ring and the regenerated catalyst C went to the next
circle [Scheme 22B]. It is important to note that no literature report regarding the catalytic asymmetric
synthesis of such a molecule was available. Thus, it should be a great research potential for the chiral NHC-
catalyzed asymmetric reactions.
Furthermore, α-keto lactone 43 could also serve as a powerful building block in asymmetric cascade [3 + 2]
cycloaddition of α-isothiocyanato imide 46 to give the chiral spirolactone derivative 47 with structural
complexity and diversity [Scheme 23]. However, only one example was reported . Thus, it should be a
[72]
great research potential for the aldol/cyclization reaction of α-keto lactone 43.
3-Isothiocyanato butyrolactones as C-N-C synthons
The structural complexity and richness in the stereogenic centers of spirocyclic compounds are generally
important for their potentially useful pharmaceutical properties. Moreover, biological activity seems to be
enhanced by replacing oxygen atoms in spirocyclic molecules with sulfur atoms. The isothiocyanates
bearing an electron-withdrawing group at the α-position have proved to be particularly critical for the
asymmetric synthesis of spirocyclic heterocyclic compounds [73-79] . Recently, 3-isothiocyanato butyrolactone
48 could also serve as a powerful C-N-C synthon in asymmetric [3 + 2] cycloaddition to give the chiral
spirothiobenzolactone derivatives [Scheme 24].