Page 50 - Read Online
P. 50
Page 14 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
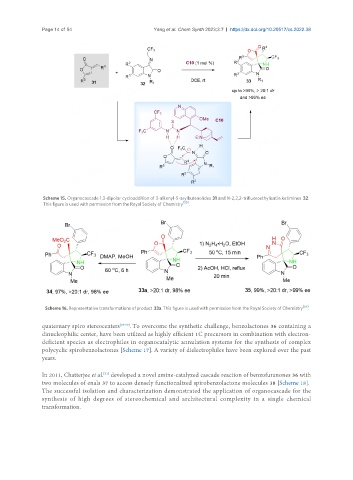
Scheme 15. Organocascade 1,3-dipolar cycloaddition of 3-alkenyl-5-arylbutenolides 31 and N-2,2,2-trifluoroethylisatin ketimines 32.
This figure is used with permission from the Royal Society of Chemistry [55] .
[55]
Scheme 16. Representative transformations of product 33a. This figure is used with permission from the Royal Society of Chemistry .
quaternary spiro stereocenters [56-59] . To overcome the synthetic challenge, benzolactones 36 containing a
dinucleophilic center, have been utilized as highly efficient 1C precursors in combination with electron-
deficient species as electrophiles in organocatalytic annulation systems for the synthesis of complex
polycyclic spirobenzolactones [Scheme 17]. A variety of dielectrophiles have been explored over the past
years.
In 2011, Chatterjee et al. developed a novel amine-catalyzed cascade reaction of benzofuranones 36 with
[51]
two molecules of enals 37 to access densely functionalized spirobenzolactone molecules 38 [Scheme 18].
The successful isolation and characterization demonstrated the application of organocascade for the
synthesis of high degrees of stereochemical and architectural complexity in a single chemical
transformation.