Page 45 - Read Online
P. 45
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 9 of 54
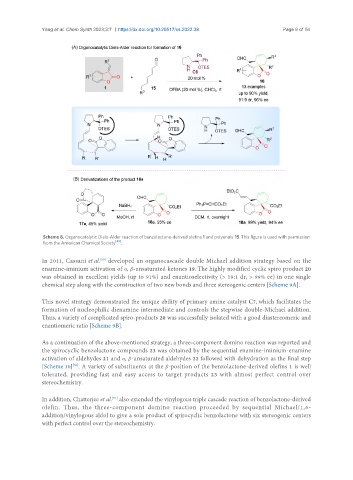
Scheme 8. Organocatalytic Diels-Alder reaction of benzolactone-derived olefins 1 and polyenals 15. This figure is used with permission
[48]
from the American Chemical Society .
In 2011, Cassani et al. developed an organocascade double Michael addition strategy based on the
[50]
enamine-iminium activation of α, β-unsaturated ketones 19. The highly modified cyclic spiro product 20
was obtained in excellent yields (up to 91%) and enantioselectivity (> 19:1 dr, > 99% ee) in one single
chemical step along with the construction of two new bonds and three stereogenic centers [Scheme 9A].
This novel strategy demonstrated the unique ability of primary amine catalyst C7, which facilitates the
formation of nucleophilic dienamine intermediate and controls the stepwise double-Michael addition.
Thus, a variety of complicated spiro-products 20 was successfully isolated with a good diastereomeric and
enantiomeric ratio [Scheme 9B].
As a continuation of the above-mentioned strategy, a three-component domino reaction was reported and
the spirocyclic benzolactone compounds 23 was obtained by the sequential enamine-iminium-enamine
activation of aldehydes 21 and α, β-unsaturated aldehydes 22 followed with dehydration as the final step
[Scheme 10] . A variety of substituents at the β-position of the benzolactone-derived olefins 1 is well
[50]
tolerated, providing fast and easy access to target products 23 with almost perfect control over
stereochemistry.
[51]
In addition, Chatterjee et al. also extended the vinylogous triple cascade reaction of benzolactone-derived
olefin. Thus, the three-component domino reaction proceeded by sequential Michael/1,6-
addition/vinylogous aldol to give a sole product of spirocyclic benzolactone with six stereogenic centers
with perfect control over the stereochemistry.