Page 42 - Read Online
P. 42
Page 6 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
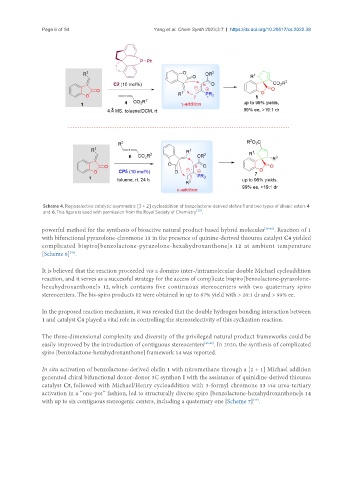
Scheme 4. Regioselective catalytic asymmetric [3 + 2] cycloaddition of benzolactone-derived olefins 1 and two types of allenic esters 4
[33]
and 6. This figure is used with permission from the Royal Society of Chemistry .
powerful method for the synthesis of bioactive natural product-based hybrid molecules [38-43] . Reaction of 1
with bifunctional pyrazolone-chromone 11 in the presence of quinine-derived thiourea catalyst C4 yielded
complicated bispiro[benzolactone-pyrazolone-hexahydroxanthone]s 12 at ambient temperature
[Scheme 6] .
[44]
It is believed that the reaction proceeded via a domino inter-/intramolecular double Michael cycloaddition
reaction, and it serves as a successful strategy for the access of complicate bispiro[benzolactone-pyrazolone-
hexahydroxanthone]s 12, which contains five continuous stereocenters with two quaternary spiro
stereocenters. The bis-spiro products 12 were obtained in up to 87% yield with > 20:1 dr and > 99% ee.
In the proposed reaction mechanism, it was revealed that the double hydrogen bonding interaction between
1 and catalyst C4 played a vital role in controlling the stereoselectivity of this cyclization reaction.
The three-dimensional complexity and diversity of the privileged natural product frameworks could be
easily improved by the introduction of contiguous stereocenters [45,46] . In 2020, the synthesis of complicated
spiro [benzolactone-hexahydroxanthone] framework 14 was reported.
In situ activation of benzolactone-derived olefin 1 with nitromethane through a [2 + 1] Michael addition
generated chiral bifunctional donor-donor 3C synthon I with the assistance of quinidine-derived thiourea
catalyst C5, followed with Michael/Henry cycloaddition with 3-formyl chromone 13 via urea-tertiary
activation in a ‘‘one-pot’’ fashion, led to structurally diverse spiro [benzolactone-hexahydroxanthone]s 14
[47]
with up to six contiguous stereogenic centers, including a quaternary one [Scheme 7] .